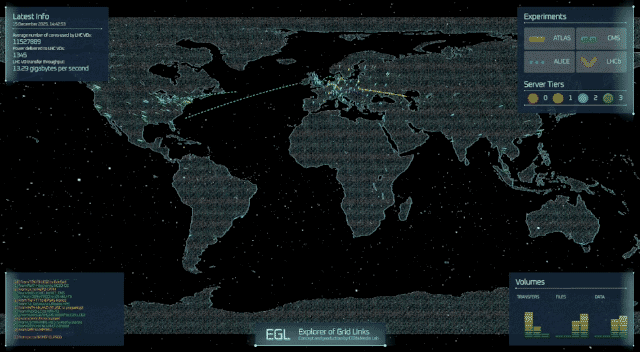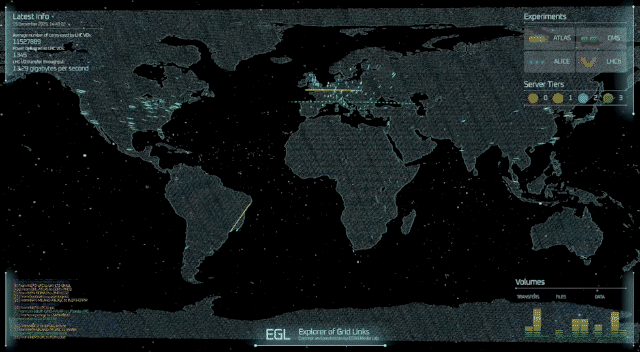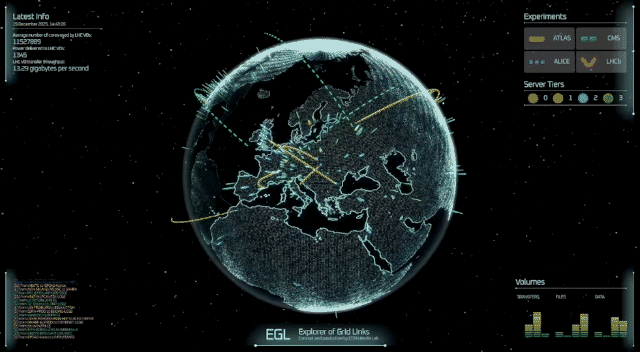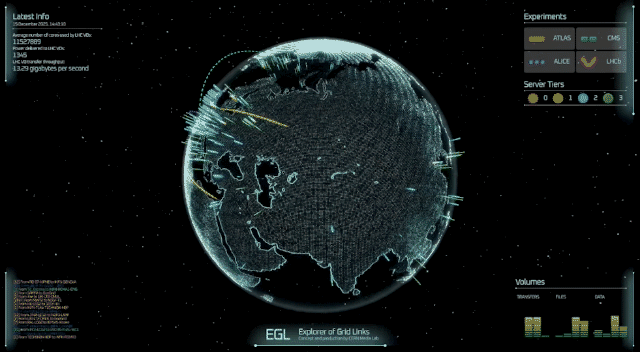
A professor at the University of Cincinnati and his colleagues have figured out something two of America’s most famous fictional physicists couldn’t: how to theoretically produce subatomic particles called axions in fusion reactors.
Particle physicists Sheldon Cooper and Leonard Hofstadter, roommates in the sitcom “The Big Bang Theory,” worked on the problem in three episodes of Season 5, but couldn’t crack it.
Now UC physics Professor Jure Zupan and his theoretical physicist co-authors at the Fermi National Laboratory, MIT and Technion–Israel Institute of Technology think they have one solution in a study published in the Journal of High Energy Physics.