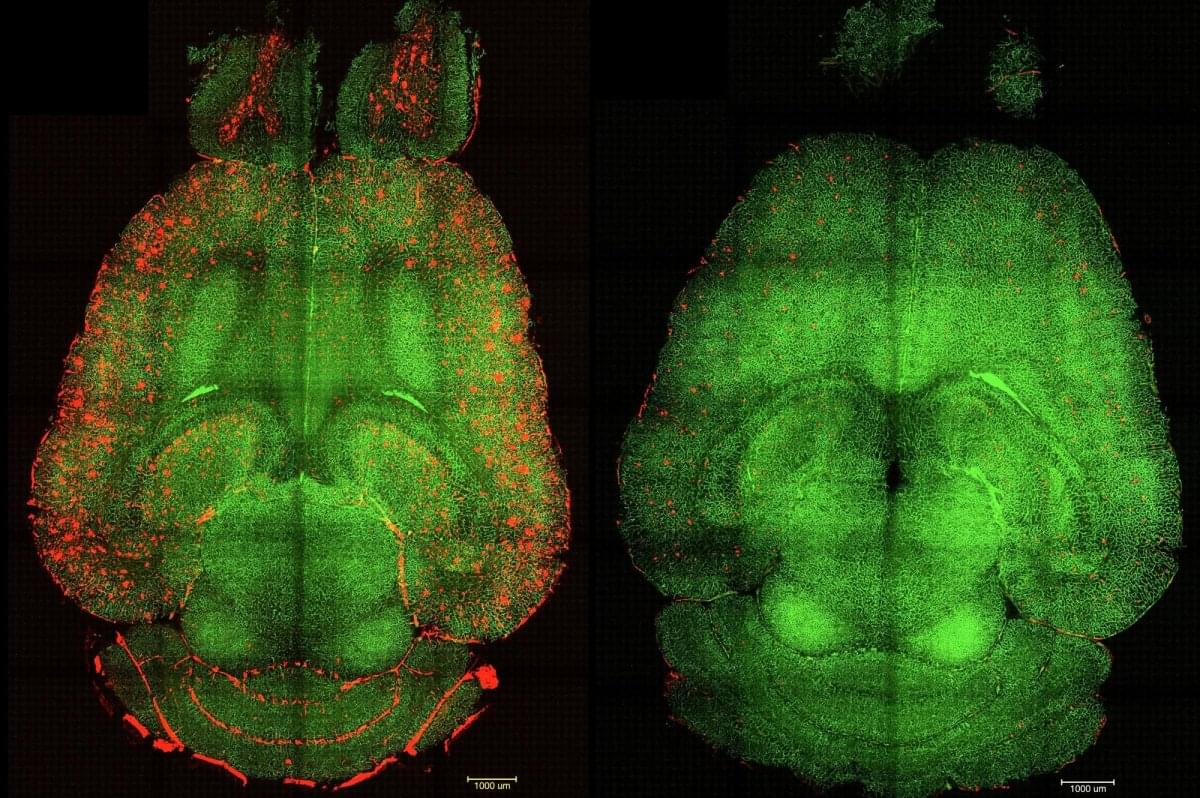
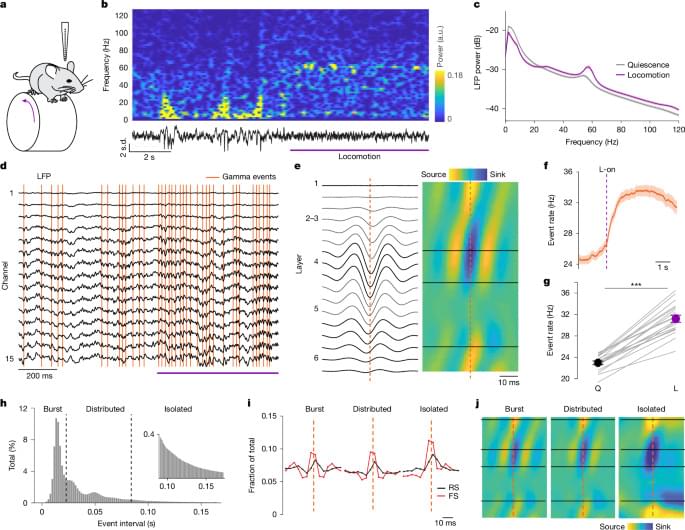
Using a new analytical method for tracking gamma band events in mouse visual cortex, flexible encoding of visual information according to behavioural context is shown.

The prevalence of Alzheimer’s disease (AD) is approximately two times higher in African Americans (AA) compared to white/European-ancestry (EA) individuals living in the U.S. Some of this is due to social determinants of health such as disparities in health care access and quality of education, biases in testing and higher rates of AD risk factors such as cardiovascular disease and diabetes in those who identify as African American.
Although many studies have examined differences in gene expression (a measure of the amount of protein encoded by a gene) in brain tissue from AD cases and controls in EA or mixed ancestry cohorts, the number of AA individuals in these studies was unspecified or too small to identify significant findings within this group alone.
In the largest AD study conducted in brain tissue from AA donors, researchers from Boston University Chobanian & Avedisian School of Medicine have identified many genes, a large portion of which had not previously been implicated in AD by other genetic studies, to be significantly more or less active in tissue from AD cases compared to controls. The most notable finding was a 1.5 fold higher level of expression of the ADAMTS2 gene in brain tissue from those with autopsy-confirmed AD.

People who sleep poorly are more likely than others to have brains that appear older than they actually are. This is according to a comprehensive brain imaging study from Karolinska Institutet, published in the journal eBioMedicine. The paper is titled “Poor sleep health is associated with older brain age: the role of systemic inflammation.”
Increased inflammation in the body may partly explain the association.
Poor sleep has been linked to dementia, but it is unclear whether unhealthy sleep habits contribute to the development of dementia or whether they are rather early symptoms of the disease.

Functional near-infrared spectroscopy (fNIRS) is a promising non-invasive neuroimaging technique that works by detecting changes in blood oxygenation linked to neural activity using near-infrared light. Compared to fMRI and various other methods commonly used to study the brain, fNIRS is easier to apply outside of laboratory settings.
This technique requires study participants to wear a special cap fitted with optodes, which consist of light sources that emit near-infrared light into the scalp and detectors that measure the light that is reflected back. These measurements can be used to estimate blood oxygenation in the brain’s outer layers. Despite its potential for conducting research in everyday settings, the quality of signals collected using fNIRS is known to be influenced by biophysical factors.
A team of researchers at Boston University recently set out to better delineate the extent to which people’s hair and skin color, age and sex impact the quality of fNIRS signals picked up from their scalp.

Researchers led by Aurore Perrault at Concordia University, Canada and Valeria Kebets at McGill University, Canada, have used a complex data-driven analysis to uncover relationships among multiple aspects of sleep and individual variation in health, cognition, and lifestyle.
Published in PLOS Biology, the study reveals five sleep –biopsychosocial profiles and their associated patterns of functional connectivity among brain regions.
Most studies of sleep focus on a single aspect, such as duration, and examine how it relates to a single outcome, like poor mental health. However, trying to understand and predict outcomes by combining the results of many different single-association studies invariably fails. The new study by Perrault and team takes a different approach. Using a sample of 770 people from the Human Connectome Project dataset, they conducted a multivariate, data-driven analysis.
Important genetic differences in how females and males experience depression have been revealed for the first time in findings that could pave the way for more targeted intervention and treatments.
In the study, published in Nature Communications, scientists found that genetic factors contribute more to depression risk in females than in males. The team discovered about twice as many genetic “flags” for depression in the DNA of females as they did in that of males.
“We already know that females are twice as likely to suffer from depression in their lifetime than males,” said Dr. Brittany Mitchell, Senior Researcher at QIMR Berghofer’s Genetic Epidemiology Lab. “And we also know that depression looks very different from one person to another. Until now, there hasn’t been much consistent research to explain why depression affects females and males differently, including the possible role of genetics.”
Researchers recently discovered that eight different psychiatric conditions share a common genetic basis.
A study published this year pinpointed specific variants among those shared genes and shows how they behave during brain development.
The US team found many of these variants remain active for extended periods, potentially influencing multiple developmental stages – and offering new targets for treatments that could address several disorders at once.