Sorry, we’re having trouble playing this video.


Mitochondrial Quality Control (Mitophagy), CNS Disorders, and Aging — Dr. Spring Behrouz, Ph.D., CEO, Vincere Biosciences Inc. / CEO, Neuroinitiative LLC.
Dr. Bahareh (Spring) Behrouz, PhD, is the CEO of Vincere Biosciences Inc (https://vincerebio.com/), a biotech company focused on developing novel, small molecule therapeutics targeting mitochondrial pathways and the improvement of mitochondrial quality.
Dr. Behrouz is also the CEO of NeuroInitiative, LLC (https://www.neuroinitiative.com/), a computational biology company she co-founded in 2014, which develops simulations of disease using their patented software platform. A core focus of her research at NeuroInitiative is on the elucidation of complex, converging pathways that contribute to the pathogenesis of Parkinson’s disease (PD), a neuro-degenerative brain disorder which dramatically effects movement, which nearly one million people in the U.S. are living with, and 10 million patients worldwide.
Dr. Behrouz received her graduate training at Michigan State University in the laboratory of Dr. John Goudreau and studied differential susceptibility of dopaminergic neuron sub-types in models of PD. She completed her post-doctoral training in the laboratory of Dr. Matthew Farrer at the Mayo Clinic in Jacksonville, where she primarily focused on in-vivo and primary culture models of LRRK2-mediated pathogenesis and was part of the team that discovered a new pathogenic mutation in VPS35.

Scientists at Cambridge and Leeds have successfully reversed age-related memory loss in mice and say their discovery could lead to the development of treatments to prevent memory loss in people as they age.
In a study published today in Molecular Psychiatry, the team show that changes in the extracellular matrix of the brain — ‘scaffolding’ around nerve cells—lead to loss of memory with aging, but that it is possible to reverse these using genetic treatments.
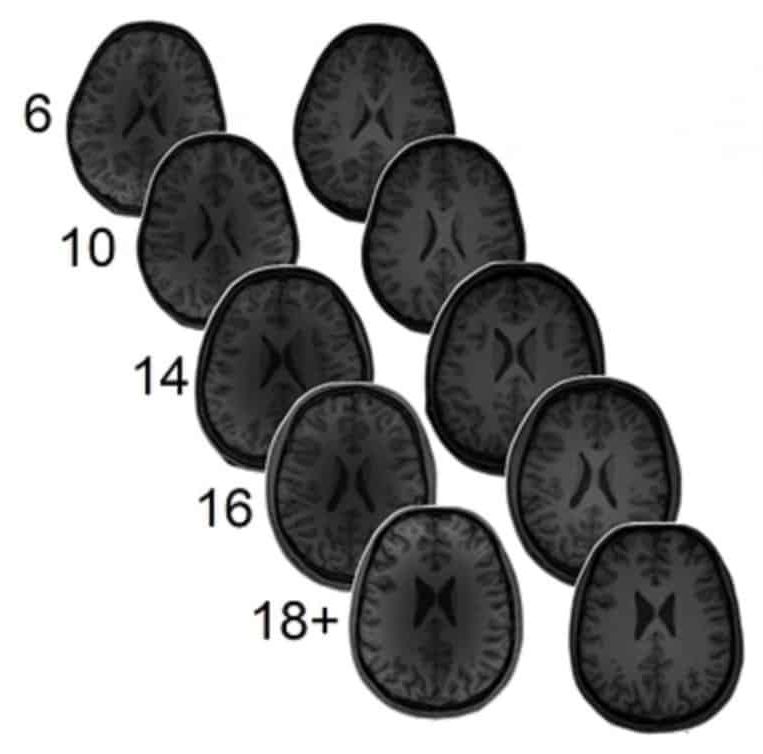
Recent evidence has emerged of the role of perineuronal nets (PNNs) in neuroplasticity—the ability of the brain to learn and adapt—and to make memories. PNNs are cartilage-like structures that mostly surround inhibitory neurons in the brain. Their main function is to control the level of plasticity in the brain. They appear at around five years old in humans, and turn off the period of enhanced plasticity during which the connections in the brain are optimized. Then, plasticity is partially turned off, making the brain more efficient but less plastic.
Summary: A new study found a person’s math ability was linked to levels of GABA and glutamate in the brain. In children, greater math fluency was associated with higher GABA levels in the left intraparietal sulcus, while lower levels of GABA were linked to math ability in adults. The reverse was true for glutamate in both children and adults.
Source: PLOS
The neurotransmitters GABA and glutamate have complementary roles — GABA inhibits neurons, while glutamate makes them more active.
“What is exciting about this is that although our study was only in mice, the same mechanism should operate in humans – the molecules and structures in the human brain are the same as those in rodents,” says Fawcett. “This suggests that it may be possible to prevent humans from developing memory loss in old age.”
An intriguing new study from researchers in the United Kingdom is proposing an innovative method to treat age-related memory loss. The preclinical research shows memory decline in aging mice can be reversed by manipulating the composition of structures in the brain known as perineuronal nets.
Perineuronal nets (PNNs) are structures in the brain that envelop certain subsets of neurons, helping stabilize synaptic activity. They essentially put the brakes on the neuroplasticity seen in the first few years of life.
Although PNNs are vital to the effective functioning of a mature adult brain, by their very nature they also limit future neural plasticity and adaptability. A new wave of research is beginning to investigate ways to modulate PNNs in adult brains in the hope of treating a variety of diseases from diabetes to post-traumatic stress disorder (PTSD).
Nature always finds a way…so they say! But it looks like it may actually be true in the case of our global plastic waste dilemma. Genetic mutations have been discovered in specific natural bacteria that enable them to break the polymer chains of certain plastics. Where have we found these bacteria? Well…in plastic recycling dumps of course. So, gloves and masks on everyone. We’re going in!
Video Transcripts available at our website.
http://www.justhaveathink.com.
Help support this channels independence at http://www.patreon.com/justhaveathink.
Or with a donation via Paypal by clicking here.
https://www.paypal.com/cgi-bin/webscr?cmd=_s-xclick&hosted_b…source=url.
You can also help keep my brain ticking over during the long hours of research and editing via the nice folks at BuyMeACoffee.com.
https://www.buymeacoffee.com/justhaveathink.
Download the Just Have a Think App from the AppStore or Google Play.
One of the most important open questions in science is how our consciousness is established. In the 1990s, long before winning the 2020 Nobel Prize in Physics for his prediction of black holes, physicist Roger Penrose teamed up with anaesthesiologist Stuart Hameroff to propose an ambitious answer.
They claimed that the brain’s neuronal system forms an intricate network and that the consciousness this produces should obey the rules of quantum mechanics —the theory that determines how tiny particles like electrons move around. This, they argue, could explain the mysterious complexity of human consciousness.
Penrose and Hameroff were met with incredulity. Quantum mechanical laws are usually only found to apply at very low temperatures. Quantum computers, for example, currently operate at around -272°C. At higher temperatures, classical mechanics takes over. Since our body works at room temperature, you would expect it to be governed by the classical laws of physics. For this reason, the quantum consciousness theory has been dismissed outright by many scientists—though others are persuaded supporters.
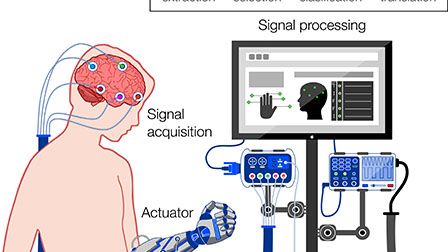
Brain–computer interfaces (BCIs) provide bidirectional communication between the brain and output devices that translate user intent into function. Among the different brain imaging techniques used to operate BCIs, electroencephalography (EEG) constitutes the preferred method of choice, owing to its relative low cost, ease of use, high temporal resolution, and noninvasiveness. In recent years, significant progress in wearable technologies and computational intelligence has greatly enhanced the performance and capabilities of EEG-based BCIs (eBCIs) and propelled their migration out of the laboratory and into real-world environments. This rapid translation constitutes a paradigm shift in human–machine interaction that will deeply transform different industries in the near future, including healthcare and wellbeing, entertainment, security, education, and marketing. In this contribution, the state-of-the-art in wearable biosensing is reviewed, focusing on the development of novel electrode interfaces for long term and noninvasive EEG monitoring. Commercially available EEG platforms are surveyed, and a comparative analysis is presented based on the benefits and limitations they provide for eBCI development. Emerging applications in neuroscientific research and future trends related to the widespread implementation of eBCIs for medical and nonmedical uses are discussed. Finally, a commentary on the ethical, social, and legal concerns associated with this increasingly ubiquitous technology is provided, as well as general recommendations to address key issues related to mainstream consumer adoption.
When will Neuralink be available to healthy individuals? It’s difficult to find a coherent train of thought pertaining to this question specifically.
Recently, I’ve started thinking more in terms of regulatory approval rather than rough timeline estimates. This sent me down a fascinating path learning more about how medical devices in general make it through “the system.”
This video essay hopefully ties together that research in an accessible way that addresses the topic more directly.
Cheers.
Andrew.
To quickly express learning and memory genes, brain cells snap both strands of DNA in many more places and cell types than previously realized, a new study shows.
The urgency to remember a dangerous experience requires the brain to make a series of potentially dangerous moves: Neurons and other brain cells snap open their DNA in numerous locations — more than previously realized, according to a new study — to provide quick access to genetic instructions for the mechanisms of memory storage.
The extent of these DNA double-strand breaks (DSBs) in multiple key brain regions is surprising and concerning, says study senior author Li-Huei Tsai, Picower Professor of Neuroscience at MIT and director of The Picower Institute for Learning and Memory, because while the breaks are routinely repaired, that process may become more flawed and fragile with age. Tsai’s lab has shown that lingering DSBs are associated with neurodegeneration and cognitive decline and that repair mechanisms can falter.