New research shows that the prefrontal cortex doesn’t simply broadcast generic commands to sensory regions—it sends finely tailored signals that shape how the brain processes vision depending on arousal and movement.

A new pill for treating dementia is delivering promising “topline” results in early-stage clinical trials, according to a recent press release by its makers.
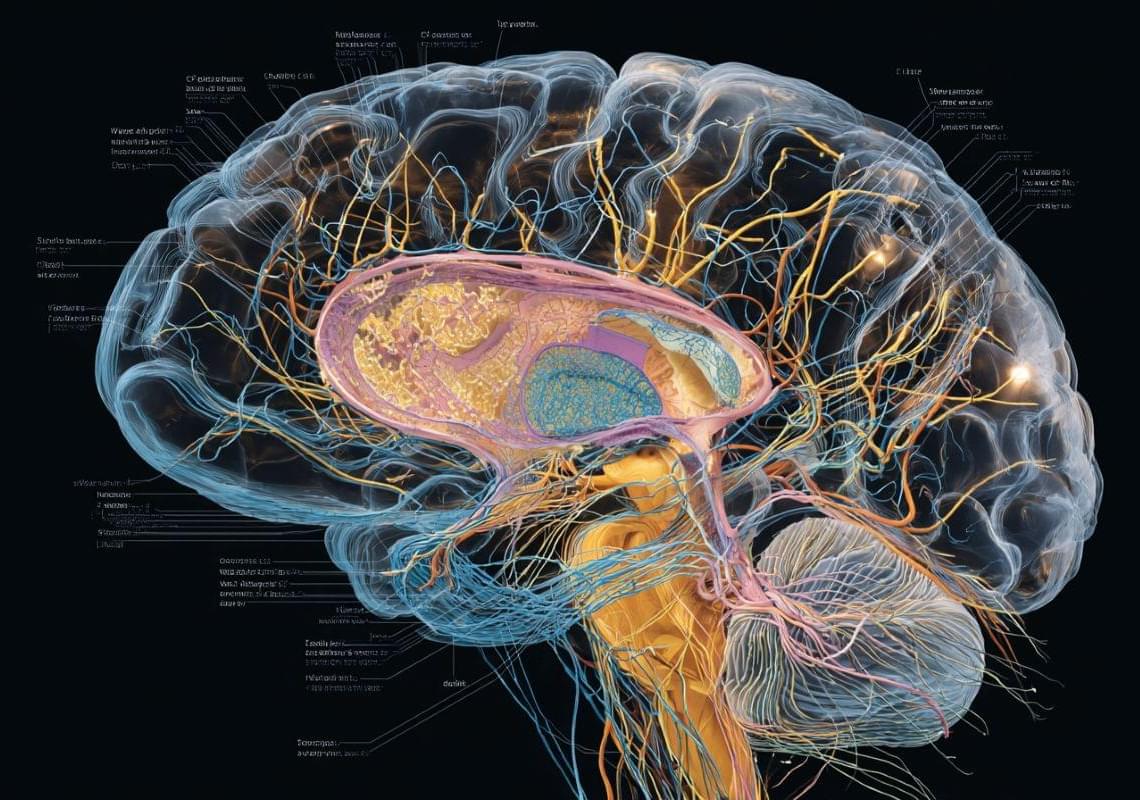
The treatment, called VES001 after its developer Vesper Bio, is designed to tackle frontotemporal dementia (FTD) – the most common type of dementia in the under-60s.
In a two-part preliminary safety trial at two medical centres in the Netherlands and the UK, VES001 was given to people showing no signs of FTD, including six volunteers with an increased genetic risk for the condition.

GLP-1 drugs have been transformative for treating obesity, but about 50 percent of patients who were prescribed this treatment ended up stopping due to severe side effects, such as nausea.
At the 2025 Society for Neuroscience meeting, experts presented new findings on how GLP-1 agonists’ action in the brain produced unwanted side effects and how these discoveries can guide future research.
Read more.
At the 2025 Society for Neuroscience meeting, scientists discussed the adverse side effects of GLP-1 agonists and new directions for future research.
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by differences in communication, behavior and the processing of sensory information. Past research has shown that some individuals diagnosed with ASD exhibit specific genetic variants or differences in the regulation of genes.
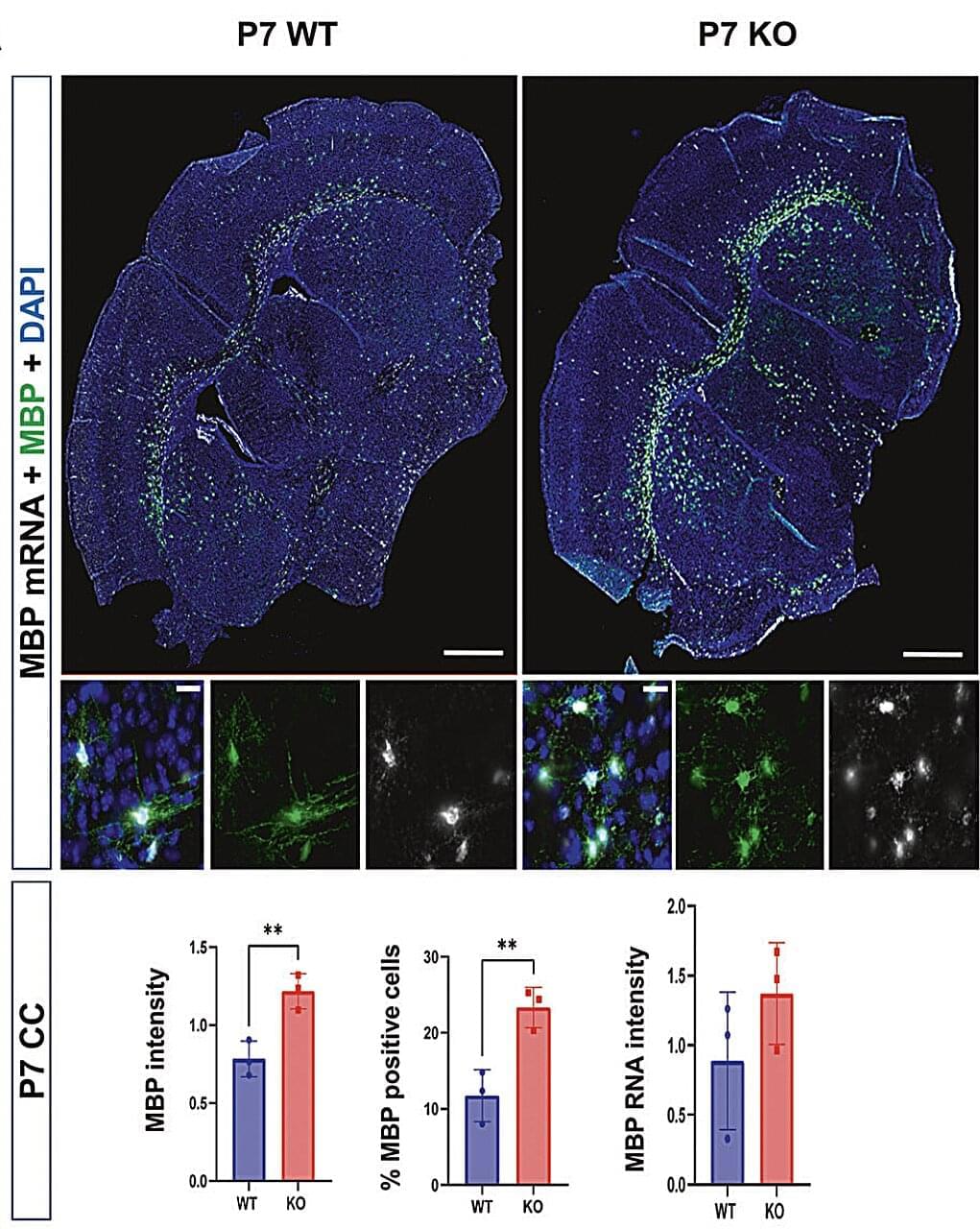
In some patients, the Shank3 gene was found to be mutated, partially or fully deleted, or not expressed as much. This gene is known to support the creation of junctions at which connected neurons communicate with each other, known as synapses.
Past findings suggest that people diagnosed with ASD who exhibit variants in Shank3 also present abnormalities in the volume, structure and function of white matter. White matter is a brain region filled with a fatty substance known as myelin, which insulates nerves and allows signals to travel faster within the nervous system.

Scientists found that reduced ATP signaling in the hippocampus can trigger both depression and anxiety in mice.
Lower ATP levels and a drop in connexin 43 expression appeared to make stressed animals more vulnerable. Manipulating this protein alone was enough to produce mood-related symptoms, while restoring it reversed them.
ATP Signaling and Mood Disorders.


Scientists use supercomputer that can process quadrillions of calculations per second to simulate mouse cortex for “virtual experiments”