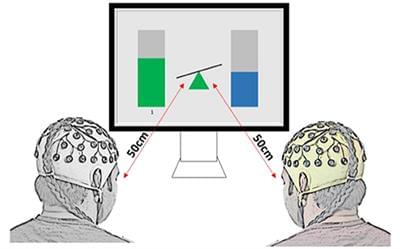
EEG hyperscanning during multiuser gaming offers opportunities to study brain characteristics of social interaction under various paradigms. In this study, we aimed to characterize neural signatures and phase-based functional connectivity patterns of gaming strategies during collaborative and competitive alpha neurofeedback games. Twenty pairs of participants with no close relationship took part in three sessions of collaborative or competitive multiuser neurofeedback (NF), with identical graphical user interface, using Relative Alpha (RA) power as a control signal. Collaborating dyads had to keep their RA within 5% of each other for the team to be awarded a point, while members of competitive dyads scored points if their RA was 10% above their opponent’s. Interbrain synchrony existed only during gaming but not during baseline in either collaborative or competitive gaming. Spectral analysis and interbrain connectivity showed that in collaborative gaming, players with higher resting state alpha content were more active in regulating their RA to match those of their partner. Moreover, interconnectivity was the strongest between homologous brain structures of the dyad in theta and alpha bands, indicating a similar degree of planning and social exchange. Competitive gaming emphasized the difference between participants who were able to relax and, in this way, maintain RA, and those who had an unsuccessful approach. Analysis of interbrain connections shows engagement of frontal areas in losers, but not in winners, indicating the formers’ attempt to mentalise and apply strategies that might be suitable for conventional gaming, but inappropriate for the alpha neurofeedback-based game. We show that in gaming based on multiplayer non-verbalized NF, the winning strategy is dependent on the rules of the game and on the behavior of the opponent. Mental strategies that characterize successful gaming in the physical world might not be adequate for NF-based gaming.
Humans are social creatures whose behavior and consciousness are heavily shaped by their environment. Hence, it is natural that hyperscanning, a technique which involves simultaneous recording of physiological activity from more than one subject, is used to deepen our understanding of human interaction. In recent years, hyperscanning has been applied to brain activity to shed light on the neurophysiological representation of various types of interpersonal communication. These range from verbal interaction (Pérez et al., 2017; Ahn et al., 2018), leader-imitator (Dumas et al., 2010; Yun et al., 2012), joint attention and joint decision-making (Toppi et al., 2016; Hu et al., 2018), to teaching or playing music in a duet (Sänger et al., 2012; Müller et al., 2013). Moreover, the neurological coupling of mothers and their infants was investigated for positive and negative emotions and their regulation (Reindl et al., 2018; Santamaria et al., 2020).