Discusses models, training neural nets, embeddings, tokens, transformers, language syntax.

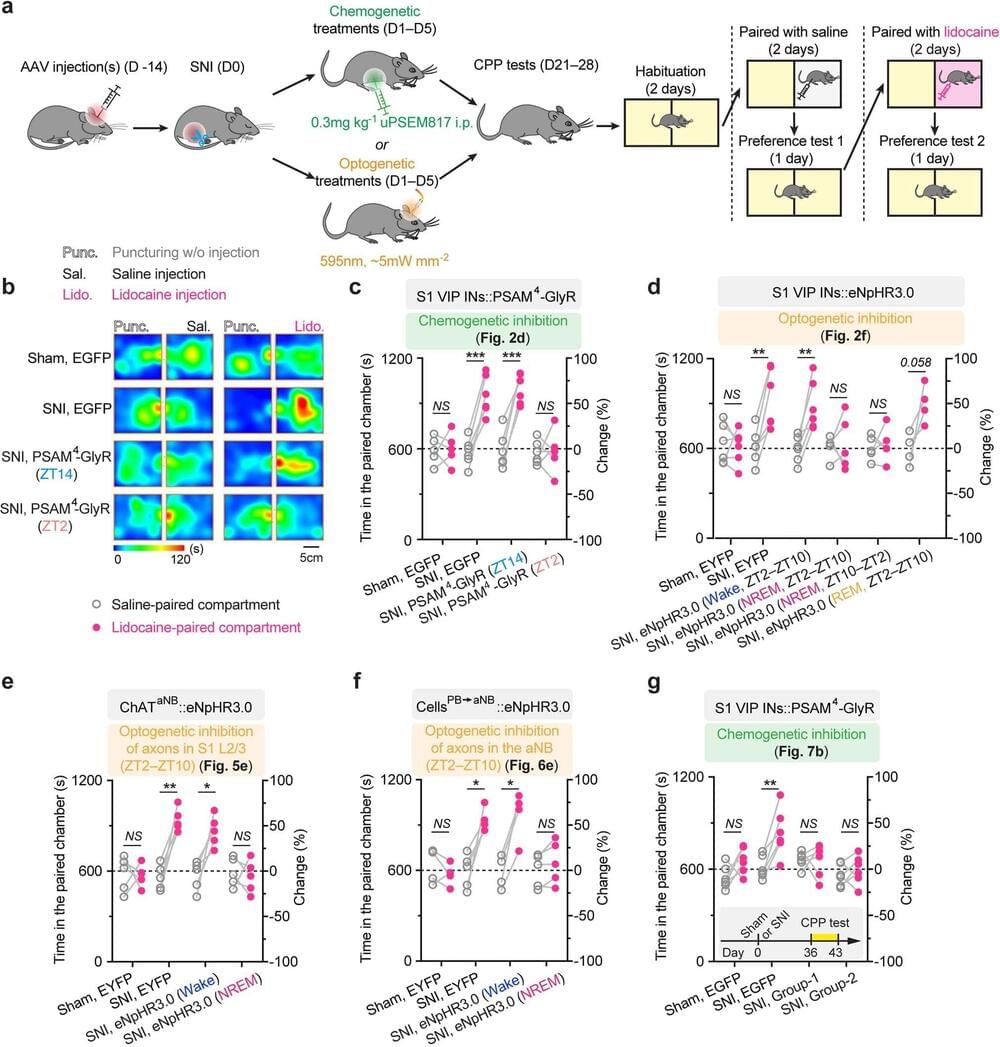
A team of neuroscientists at the New York University School of Medicine has found a link between chronic pain and overactive pyramidal neurons during sleep periods. In their study, published in the journal Nature Neuroscience, the group conducted experiments with injured mice experiencing chronic pain.
Prior research has shown that there is often a link between chronic pain and insomnia. After experiencing a neural injury of some sort, many patients are left with some degree of lasting pain. This tends to result in poor sleep and sometimes insomnia. Once that happens, the pain becomes worse, and over time becomes chronic. But why this happens has been a mystery. In this new effort, the team in New York conducted experiments with mice hoping to find the answer.
The work involved inducing chronic pain in mice by damaging two of the three branches that make up a group of sciatic nerves. Doing so led to skin sensitivity in the legs. The researchers scanned the brains of each of the mice before and after the damage. They observed that pyramidal neurons in the part of the cerebral cortex responsible for sensation processing in the skin became more active. And over the course of several weeks, the activity increased, peaking during non-REM sleep.
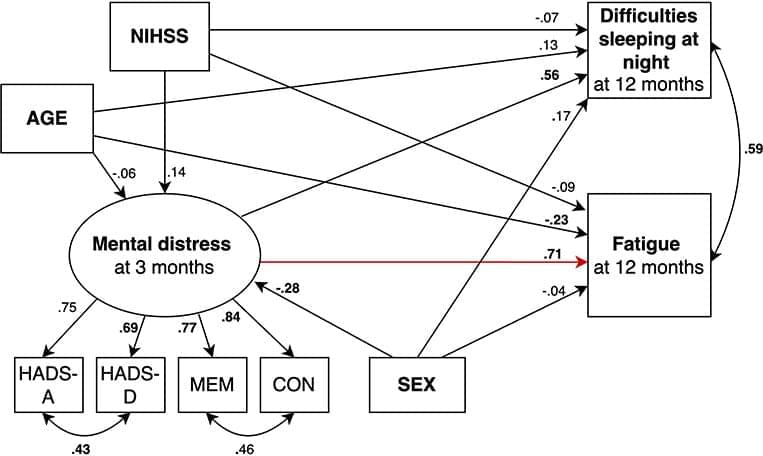
Approximately 9,000 people are admitted to Norwegian hospitals with stroke each year. About half of these patients feel exhausted afterwards, and many patients sleep more during the day than before the stroke. These after-effects are challenging and significantly affect patients’ everyday life.
However, we still have a limited understanding of which factors lead to increased fatigue and daytime sleep after stroke. Our research group therefore wanted to investigate whether cognitive and emotional complaints are related to increased fatigue and sleep during the day.
Our results were recently published in an article in the journal Frontiers in Neurology.
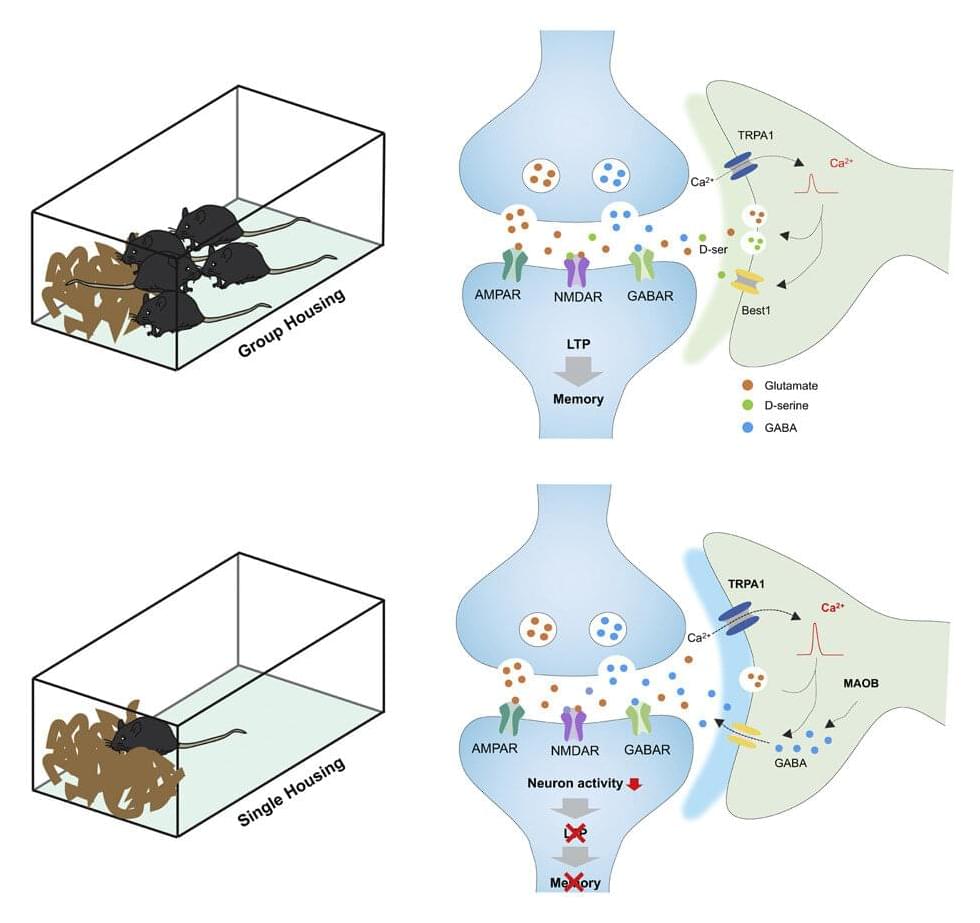
Here is an important reason to stay in touch with friends and family: social isolation causes memory and learning deficits and other behavioral changes. Many brain studies have focused on the effects social deprivation has on neurons, but little is known about the consequences for the most abundant brain cell, the astrocyte.
Researchers at Baylor College of Medicine working with animal models report in the journal Neuron that during social isolation, astrocytes become hyperactive, which in turn suppresses brain circuit function and memory formation. Importantly, inhibiting astrocyte hyperactivity reversed the cognitive deficits associated with social deprivation.
“One thing we have learned during the COVID pandemic is that social isolation can influence cognitive functions, as previous studies suggested,” said co-first author, Yi-Ting Cheng, graduate student in Dr. Benjamin Deneen’s lab at Baylor. “This motivated co-first author Dr. Junsung Woo and me to further investigate the effects of social isolation in the brain, specifically in astrocytes.”
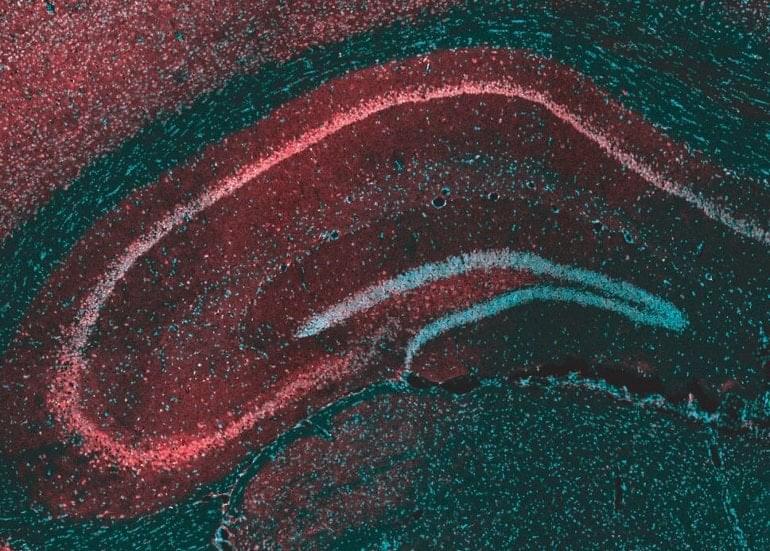
Summary: Cannabidiol, or CBD, blocks the ability of lysophosphatidylinositol (LPI) to amplify neural signals in the hippocampus. LPI weakens the signals that counter seizures, further explaining the value of CBD to treat epilepsy.
Source: NYU
A study reveals a previously unknown way in which cannabidiol (CBD), a substance found in cannabis, reduces seizures in many treatment-resistant forms of pediatric epilepsy.

Losing as little as 6–7 minutes per day to sedentary behavior or low-intensity activities has been linked to a decline in cognitive function, according to recent research.
The daily time spent in moderate and intense physical activity is linked to mid-life brain power, according to new research published in the Journal of Epidemiology & Community Health.
The results indicate that the optimal level for working memory and mental tasks, such as planning and organization, is at this intensity level. Replacing it with just 6–7 minutes of light-intensity activities or sedentary behavior per day is linked to decreased cognitive performance.

Researchers from The University of Queensland have discovered the active compound from an edible mushroom that boosts nerve growth and enhances memory.
Professor Frederic Meunier from the Queensland Brain Institute said the team had identified new active compounds from the mushroom, Hericium erinaceus. This type of edible mushroom, commonly known as the Lion’s Mane Mushroom, is native to North America, Europe, and Asia. It is commonly sought after for its unique flavor and texture, and it is also used in traditional Chinese medicine to boost the immune system and improve digestive health.
Researchers have discovered lion’s mane mushrooms improve brain cell growth and memory in pre-clinical trials.

Metas Toolformer is designed to learn to use tools independently, outperforming larger language models in certain downstream tasks.
Natural Language is the programming language of the brain, wrote science fiction author Neal Stephenson in his 1992 novel Snow Crash. Recent advances in machine processing of natural language show that language can also be the programming language of machines – as they get better at understanding it.
With “Toolformer”, Meta wants to extend this principle to the use of tools.

I notice that the token in question happens to be segmented as “_an” and “_a” and not “_an_” or “_a_”.
So continuations like [_a, moral,_fruit] or [_an, tagonist, ic,_monster, s] could be possible (assuming those are all legal tokens).
I am reminded of the wonderful little nuggest in linguistics, where people are supposed to have said something like “a narange” (because that kind of fruit came from the spanish province of “naranja”). The details on these claims are often not well documented.
Celebrated psychologist Steven Pinker explains how language provides a window to the inner workings of the human mind.