While foraging, animals including humans and monkeys are continuously making decisions about where to search for food and when to move among possible sources of sustenance.



A study from the University of Michigan has shown that traumatic experiences during childhood may get “under the skin” later in life, impairing the muscle function of people as they age.
The study examined the function of skeletal muscle of older adults paired with surveys of adverse events they had experienced in childhood. It found that people who experienced greater childhood adversity, reporting one or more adverse events, had poorer muscle metabolism later in life. The research, led by University of Michigan Institute for Social Research scientist Kate Duchowny, is published in Science Advances.
Duchowny and her co-authors used muscle tissue samples from people participating in the Study of Muscle, Mobility and Aging, or SOMMA. The study includes 879 participants over age 70 who donated muscle and fat samples as well as other biospecimens. The participants also were given a variety of questionnaires and physical and cognitive assessments, among other tests.

A recent study published in the Journal of Neuroscience Research offers an intriguing perspective on the effects of regular cannabis use. Contrary to the commonly held view that cannabis has primarily negative impacts on mental health and behavior, the study suggests that regular cannabis users may have a heightened ability to understand the emotions of others. This enhanced empathetic ability is linked to increased connectivity within certain brain regions, particularly the anterior cingulate cortex, a key area involved in processing empathy.
Cannabis is one of the most widely used psychoactive substances worldwide, yet its impact on mental health and cognitive functions remains a subject of contentious debate. Traditional research predominantly highlights the negative consequences associated with cannabis use, particularly its potential to impair cognitive functions and contribute to mental health issues.
These studies often focus on how cannabis interacts with brain regions like the anterior cingulate cortex, which is known to have a high density of cannabinoid receptors and plays a significant role in cognitive processes such as decision making and emotion regulation.
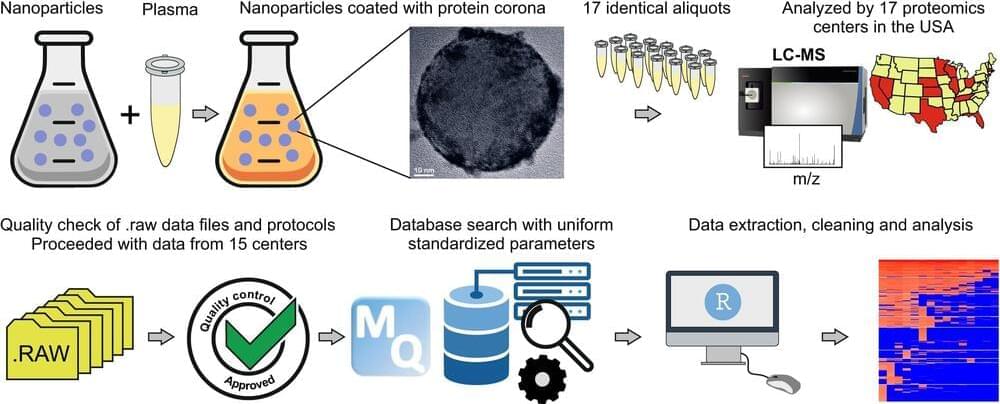
Is it possible for nanoparticles to go through the digestive system and deliver medicine directly to the brain tissue? Researchers from Michigan State University say yes, and their latest findings are expected to benefit patients with neurodegenerative disorders like multiple sclerosis, or MS; amyotrophic lateral sclerosis, or ALS; and Parkinson’s disease, or PD.

Saddened by the news of Prof. Daniel Dennett’s passing. a brilliant philosopher with with such great influence in cognitive science. It’s such a great loss.
Daniel Dennett, professor emeritus of philosophy at Tufts University, well-known for his work in philosophy of mind and a wide range of other philosophical areas, has died.
Professor Dennett wrote extensively about issues related to philosophy of mind and cognitive science, especially consciousness. He is also recognized as having made significant contributions to the concept of intentionality and debates on free will. Some of Professor Dennett’s books include Content and Consciousness (1969), Brainstorms: Philosophical Essays on Mind and Psychology (1981), The Intentional Stance (1987), Consciousness Explained (1992), Darwin’s Dangerous Idea (1995), Breaking the Spell (2006), and From Bacteria to Bach and Back: The Evolution of Minds (2017). He published a memoir last year entitled I’ve Been Thinking. There are also several books about him and his ideas. You can learn more about his work here.
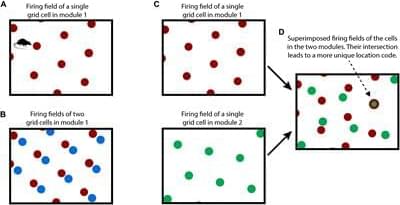
How the neocortex works is a mystery. In this paper we propose a novel framework for understanding its function. Grid cells are neurons in the entorhinal cortex that represent the location of an animal in its environment. Recent evidence suggests that grid cell-like neurons may also be present in the neocortex. We propose that grid cells exist throughout the neocortex, in every region and in every cortical column. They define a location-based framework for how the neocortex functions. Whereas grid cells in the entorhinal cortex represent the location of one thing, the body relative to its environment, we propose that cortical grid cells simultaneously represent the location of many things. Cortical columns in somatosensory cortex track the location of tactile features relative to the object being touched and cortical columns in visual cortex track the location of visual features relative to the object being viewed. We propose that mechanisms in the entorhinal cortex and hippocampus that evolved for learning the structure of environments are now used by the neocortex to learn the structure of objects. Having a representation of location in each cortical column suggests mechanisms for how the neocortex represents object compositionality and object behaviors. It leads to the hypothesis that every part of the neocortex learns complete models of objects and that there are many models of each object distributed throughout the neocortex. The similarity of circuitry observed in all cortical regions is strong evidence that even high-level cognitive tasks are learned and represented in a location-based framework.
The human neocortex learns an incredibly complex and detailed model of the world. Each of us can recognize 1000s of objects. We know how these objects appear through vision, touch, and audition, we know how these objects behave and change when we interact with them, and we know their location in the world. The human neocortex also learns models of abstract objects, structures that don’t physically exist or that we cannot directly sense. The circuitry of the neocortex is also complex. Understanding how the complex circuitry of the neocortex learns complex models of the world is one of the primary goals of neuroscience.
Vernon Mountcastle was the first to propose that all regions of the neocortex are fundamentally the same. What distinguishes one region from another, he argued, is mostly determined by the inputs to a region and not by differences in intrinsic circuitry and function. He further proposed that a small volume of cortex, a cortical column, is the unit of replication (Mountcastle, 1978). These are compelling ideas, but it has been difficult to identify what a column could do that is sufficient to explain all cognitive abilities. Today, the most common view is that the neocortex processes sensory input in a series of hierarchical steps, extracting more and more complex features until objects are recognized (Fukushima, 1980; Riesenhuber and Poggio, 1999).

A new study published in the Journal of Neuroscience indicates that the sense of smell is significantly influenced by cues from other senses, whereas the senses of sight and hearing are much less affected.
A popular theory of the brain holds that its main function is to predict what will happen next, so it reacts mostly to unexpected events. Most research on this topic, called predictive coding, has only focused on what we see, but no one knows if the different senses, such as smell, work in the same way.
To figure out more about how smell relates to how we handle different sensory impressions, the researchers conducted a study with three experiments, two behavioral experiments, and one experiment using the brain imaging method fMRI at Stockholm University Brain Imaging Centre (SUBIC).
Cannabinol (CBN) is a chemical found in cannabis that exhibits milder psychoactive properties than most cannabis chemicals, though research pertaining to its medical applications remains limited. Now, a team of researchers led by The Salk Institute for Biological Studies have published a study in Redox Biology that addresses the potential for CBN to serve as a method for neurological disorders, including traumatic brain injuries, Parkinson’s disease, and Alzheimer’s disease.
For the study, the researchers produced four CBN analogs that exhibited greater neuroprotective capabilities compared to the traditional CBN molecule and tested them on Drosophila fruit flies. In the end, the researchers discovered these CBN analogs possessed neuroprotective capabilities that surpassed traditional CBN molecules, including the treating of traumatic brain injuries. While not tested during this study, these CBN analogs could be used to also treat a myriad of neurological disorders, including Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease.
“Our findings help demonstrate the therapeutic potential of CBN, as well as the scientific opportunity we have to replicate and refine its drug-like properties,” said Dr. Pamela Maher, who is a research professor in the Cellular Neurobiology Laboratory at Salk and a co-author on the study. “Could we one day give this CBN analog to football players the day before a big game, or to car accident survivors as they arrive in the hospital? We’re excited to see how effective these compounds might be in protecting the brain from further damage.”