New FDA-approved blood tests for Alzheimer’s offer a faster and less invasive diagnosis, but they’re not approved for everyone. Here’s what to know.
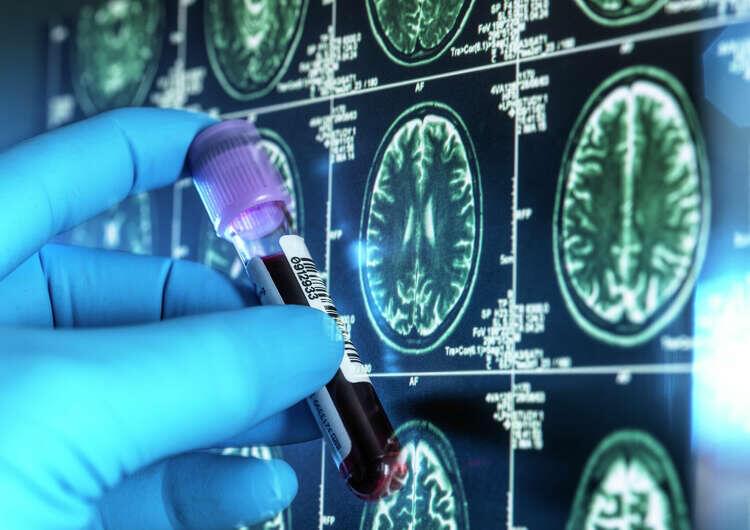

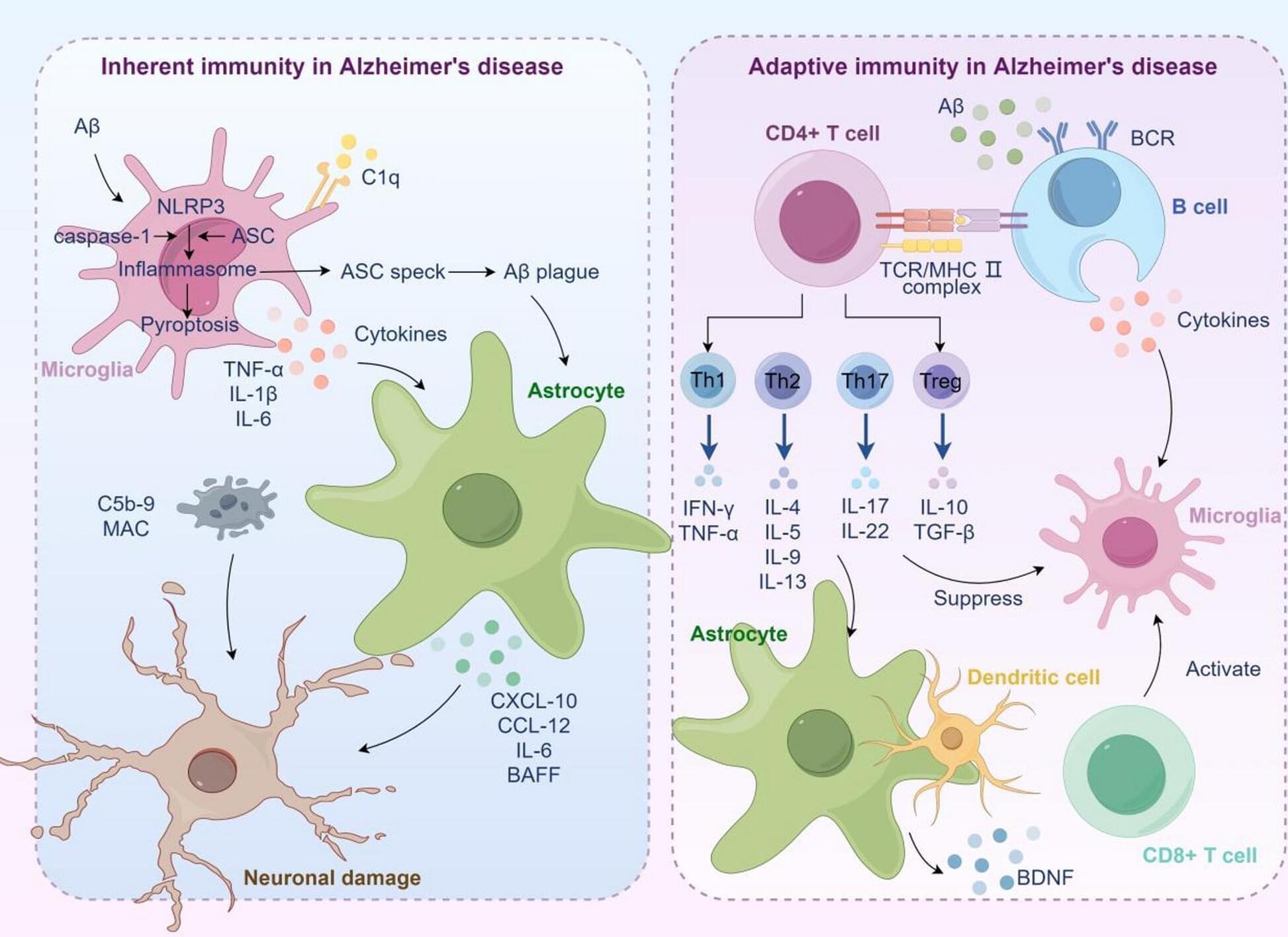
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by macroscopic features such as cortical atrophy, narrowing of the gyri, widening of the sulci, and enlargement of the ventricles. At the cellular level, the pathological characteristics include the extracellular aggregation of β-amyloid (Aβ) forming senile plaques, and the intracellular accumulation of hyperphosphorylated tau proteins forming neurofibrillary tangles. AD leads to the progressive decline of cognitive, behavioral, and social abilities, with no effective treatment available currently. The pathophysiology of AD is complex, involving mechanisms such as immune dysregulation and lipid metabolism alterations. Immune cells, such as microglia, can identify and clear pathological aggregates like Aβ early in the disease.
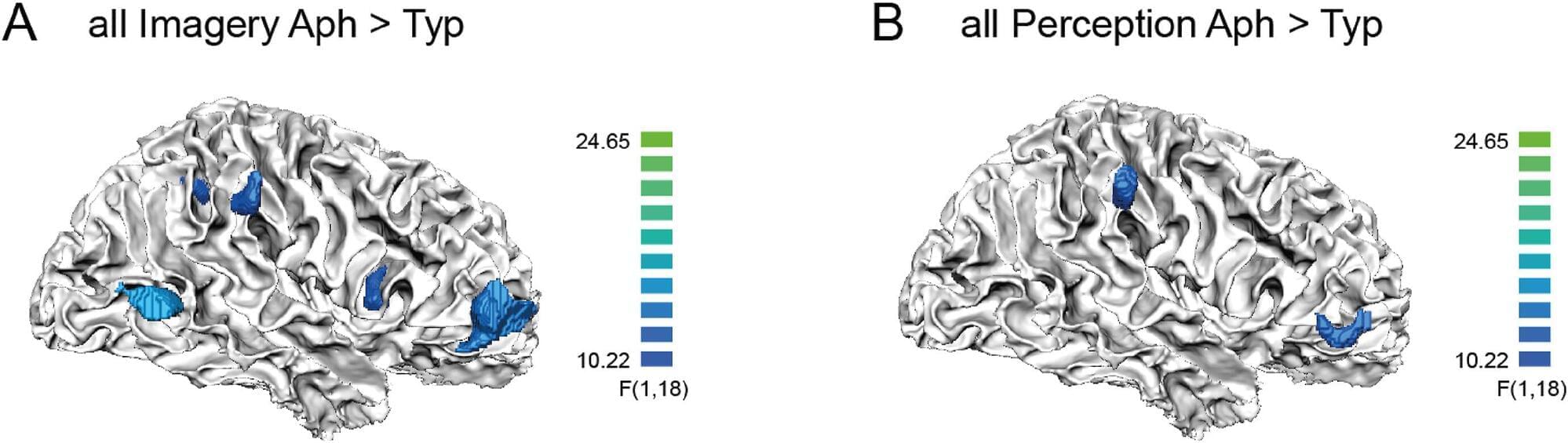
Thanks to 7T fMRI, researchers from Paris Brain Institute and NeuroSpin, the CEA’s neuroimaging center, are exploring the neural substrate of visual imagery at very high resolution for the first time. Their results, published in Cortex, pave the way for a better understanding of this fascinating cognitive ability, which some of us entirely lack.
Visual imagery—the ability to mentally summon the image of a landscape, a person, or an object that is not directly observable—varies greatly in intensity from one individual to another. Some people can recall a detailed city map and walk through each street as if watching a movie. Thinking of a loved one, others may barely make out their silhouette and hair color.
Interestingly, about 4% of the population seems completely unable to visualize a scene on demand: this is known as aphantasia, a cognitive peculiarity known for over a century but only recently studied by scientists.
Researchers publishing in Aging Cell have used single-cell transcriptomics to discover new insights into how neural stem cells (NSCs) change with aging.
Adults do generate neurons
The adult brain does generate new neurons [1], particularly in the hippocampus, the part of the brain responsible for memory formation [2]. Neurogenesis is limited to very specific niches, however, and does not occur across the entire brain [3]. This is accomplished by NSCs, cells that can differentiate into neural progenitors (NPs), which can themselves differentiate into both neurons and astrocytes and have less ability to proliferate [4]. Astrocytes are helper cells that support neurons’ connections and metabolism [5].
As research continues, the term “bird brain” no longer carries a negative connotation. Avian researcher John Marzluff showcases a few amazing, problem solving (and sometimes vindictive) feats accomplished by crows in order to break down common misconceptions about avian intelligence.
John Marzluff, Ph.D., is the James W. Ridgeway Professor of Wildlife Science at the University of Washington. His research has been the focus of articles in the New York Times, National Geographic, Audubon, Boys Life, The Seattle Times, and National Wildlife. PBS’s NATURE featured his raven research in its production, “Ravens,” and his crow research in the film documentary, “A Murder of Crows”. His graduate and initial post-doctoral research focused on the social behavior and ecology of jays and ravens. He was especially interested in communication, social organization, and foraging behavior. His current research brings this behavioral approach to pressing conservation issues including raptor management, management of pest species, and assessment of nest predation.
His book, In the Company of Crows and Ravens (with Tony Angell, 2005 Yale U. Press) blends biology, conservation, and anthropology to suggest that human and crow cultures have co-evolved. This book won the 2006 Washington State Book Award for general nonfiction. With his wife, Colleen, he has published Dog Days, Raven Nights (2011 Yale University Press), which combines reflection with biology and the recreational pursuit of dog sledding to show how a life in science blooms. Gifts of the Crow (2012 Free Press) applies a neurobiological perspective to understand the amazing feats of corvids. He is a member of the board of editors for Acta Ornithologica, Landscape Ecology and Ecological Applications. Currently leader of the U.S. Fish and Wildlife Service’s Recovery Team for the critically endangered Mariana Crow, he is also a Fellow of the American Ornithologist’s Union.
In the spirit of ideas worth spreading, TEDx is a program of local, self-organized events that bring people together to share a TED-like experience. At a TEDx event, TEDTalks video and live speakers combine to spark deep discussion and connection in a small group. These local, self-organized events are branded TEDx, where x = independently organized TED event. The TED Conference provides general guidance for the TEDx program, but individual TEDx events are self-organized.* (*Subject to certain rules and regulations)
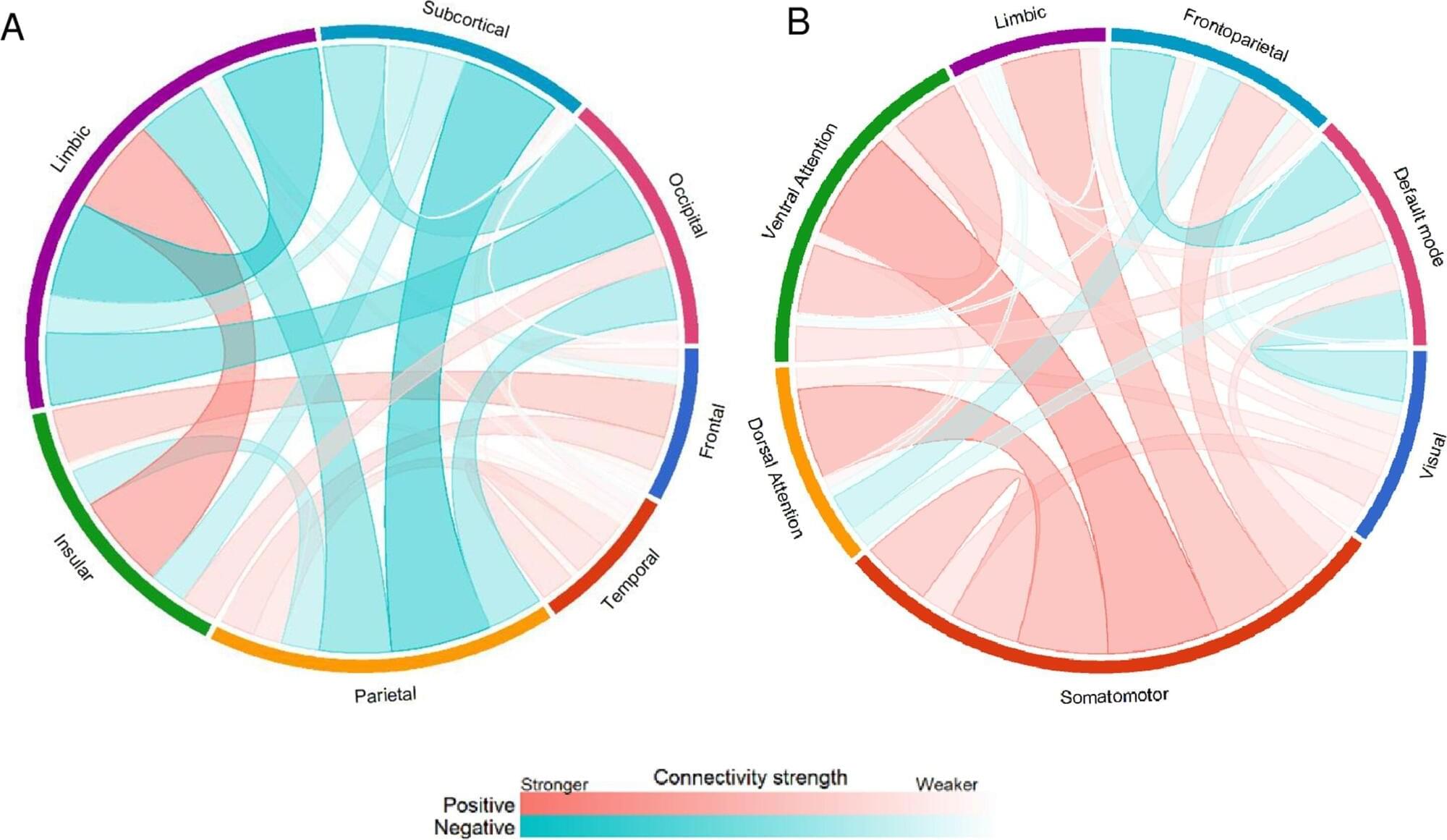
Social engagement is a vital component of psychological and physical well-being linked to better health and a longer life, yet many older adults struggle to maintain relationships that support these outcomes.
New research from Nanyang Technological University in Singapore finds that changes in the brain’s intrinsic functional connectivity networks fully account for the decline in sociability observed with aging.
Sociability is a trait encompassing communication effectiveness, emotional management, and social assertiveness, that tends to diminish with age. Older adults, particularly those who live alone, are at increased risk of isolation, limiting forms of social participation.


A new study published in JAMA Psychiatry makes the case that symptom provocation may significantly improve the clinical effectiveness of repetitive transcranial magnetic stimulation (rTMS), a noninvasive brain stimulation method used to treat depression, obsessive-compulsive disorder (OCD) and nicotine dependence.
The study was conceptualized, designed and supervised by Heather Burrell Ward, MD, assistant professor of Psychiatry and Behavioral Sciences and director of Neuromodulation Research, in collaboration with Simon Vandekar, Ph.D., associate professor of Biostatistics and Daniel Bello and Megan Jones, two students in their respective labs.
This is the first large-scale meta-analysis to examine whether deliberately triggering symptoms immediately before administering rTMS enhances treatment outcomes.