Researchers at École Polytechnique have demonstrated the first superconducting quantum circuit architecture that integrates a carbon nanotube.


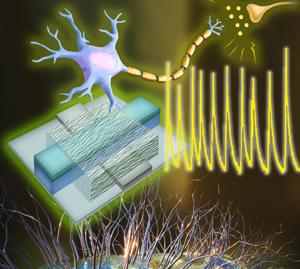
A team of engineers at the University of Massachusetts Amherst has announced the creation of an artificial neuron with electrical functions that closely mirror those of biological ones. Building on their previous groundbreaking work using protein nanowires synthesized from electricity-generating bacteria, the team’s discovery means that we could see immensely efficient computers built on biological principles which could interface directly with living cells.
“Our brain processes an enormous amount of data,” says Shuai Fu, a graduate student in electrical and computer engineering at UMass Amherst and lead author of the study published in Nature Communications. “But its power usage is very, very low, especially compared to the amount of electricity it takes to run a Large Language Model, like ChatGPT.”
The human body is over 100 times more electrically efficient than a computer’s electrical circuit. The human brain is composed of billions of neurons, specialized cells that send and receive electrical impulses all over the body. While it takes only about 20 watts for your brain to, say, write a story, a LLM might consume well over a megawatt of electricity to do the same task.
A team of engineers at the University of Massachusetts Amherst has announced the creation of an artificial neuron with electrical functions that closely mirror those of biological ones. Building on their previous groundbreaking work using protein nanowires synthesized from electricity-generating bacteria, the team’s discovery means that we could see immensely efficient computers built on biological principles which could interface directly with living cells.
“Our brain processes an enormous amount of data,” says Shuai Fu, a graduate student in electrical and computer engineering at UMass Amherst and lead author of the study published in Nature Communications. “But its power usage is very, very low, especially compared to the amount of electricity it takes to run a Large Language Model, like ChatGPT.”
The human body is over 100 times more electrically efficient than a computer’s electrical circuit. The human brain is composed of billions of neurons, specialized cells that send and receive electrical impulses all over the body. While it takes only about 20 watts for your brain to, say, write a story, a LLM might consume well over a megawatt of electricity to do the same task.

Concrete already builds our world, and now it’s one step closer to powering it, too. Made by combining cement, water, ultra-fine carbon black (with nanoscale particles), and electrolytes, electron-conducting carbon concrete (ec3, pronounced “e-c-cubed”) creates a conductive “nanonetwork” inside concrete that could enable everyday structures like walls, sidewalks, and bridges to store and release electrical energy. In other words, the concrete around us could one day double as giant “batteries.”
As MIT researchers report in a new PNAS paper, optimized electrolytes and manufacturing processes have increased the energy storage capacity of the latest ec3 supercapacitors by an order of magnitude.
In 2023, storing enough energy to meet the daily needs of the average home would have required about 45 cubic meters of ec3, roughly the amount of concrete used in a typical basement. Now, with the improved electrolyte, that same task can be achieved with about 5 cubic meters, the volume of a typical basement wall.
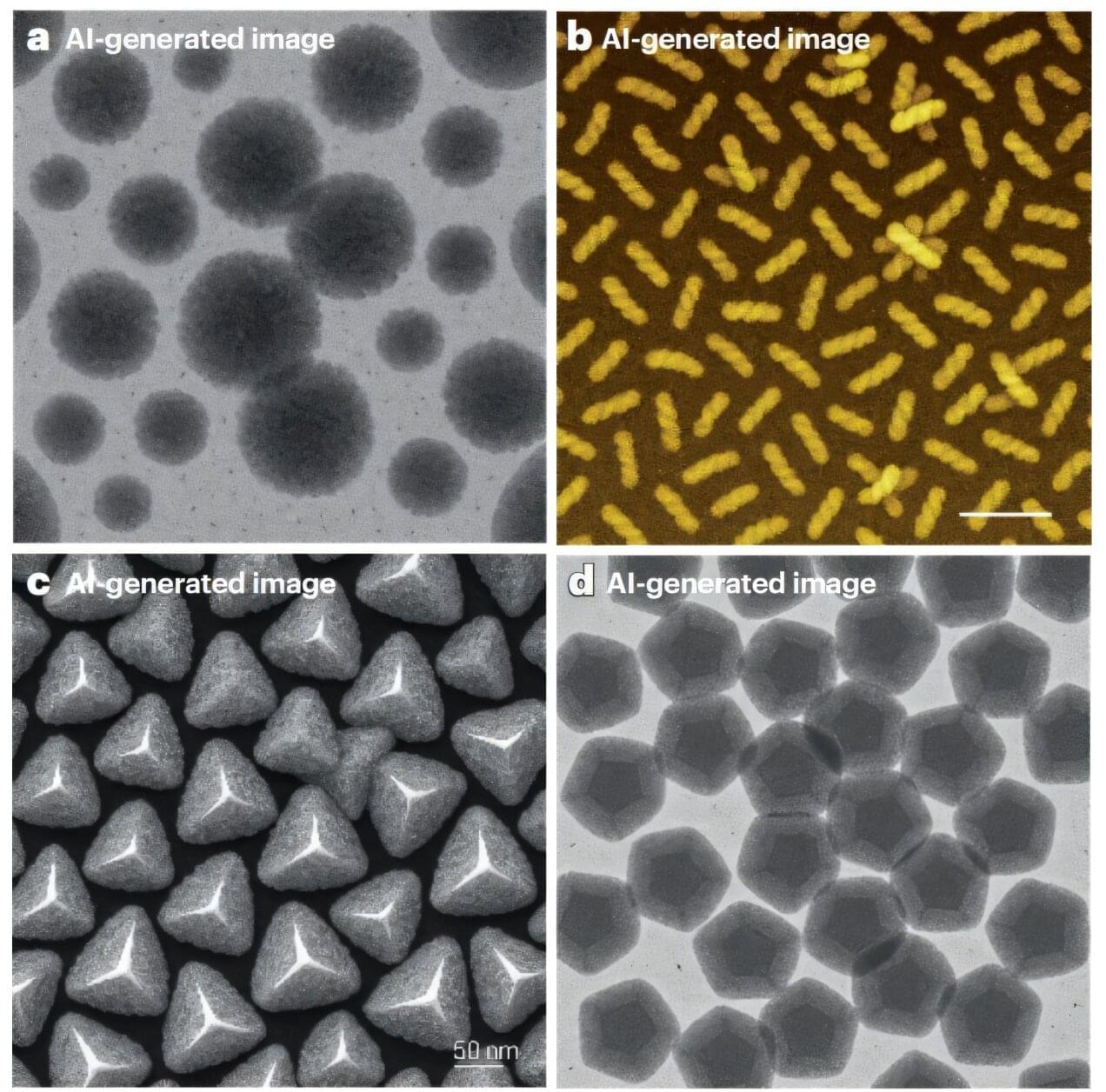
Black-and-white images of pom-pom–like clusters, semi-translucent fields of tiny dark gray stars on a pale background, and countless other abstract patterns are a familiar sight in scientific papers describing the shapes and properties of newly engineered materials.
So, when research images show particles that resemble puffed popcorn or perfectly smooth “Tic Tacs,” it might not trigger our AI suspicion radar, but researchers in a recent study caution otherwise.
Microscopy images are indispensable in nanomaterials science, as they reveal the hidden intricacies and fascinating shapes that tiny particles assume, which appear to be a pile of dust to the naked eye.
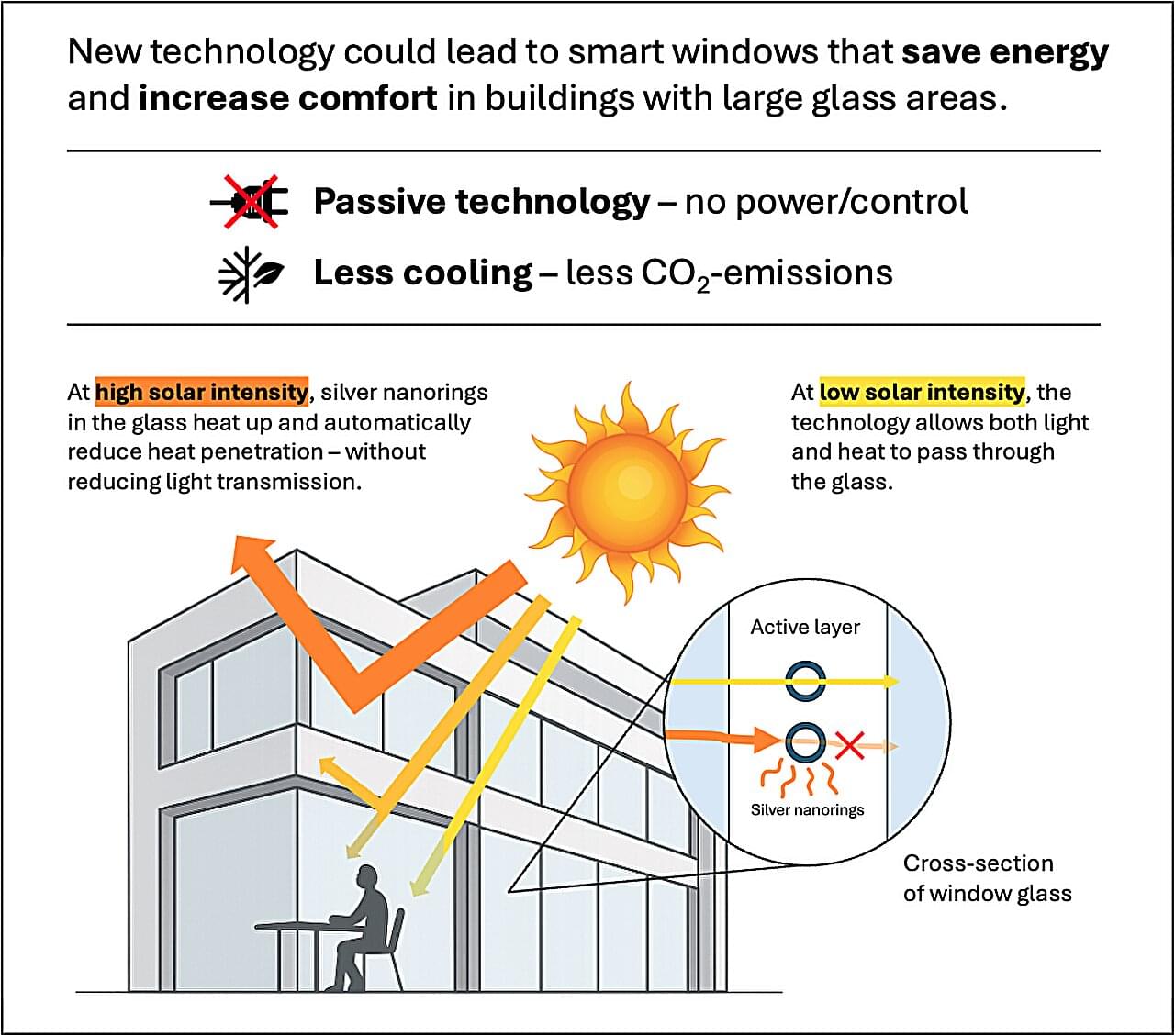
A new Danish research breakthrough could make buildings far more energy-efficient in the future. Researchers from Aarhus University’s Interdisciplinary Nanoscience Center (iNANO) have developed a light-responsive hybrid material based on so-called silver nanorings that automatically responds to solar intensity and regulates how much heat penetrates through windows.
The microscopic silver rings increasingly block near-infrared light as sunlight becomes stronger—without making the glass less transparent.
The technology functions without the use of power, sensors, or electronics—and could potentially be applied as a window coating in, for example, office buildings and modern residential buildings where large glass areas are common and heat radiation from the sun can be a challenge. This makes the solution particularly relevant at a time when energy consumption for cooling exceeds the need for heating in large parts of the world.

A team of scientists from the University of Chicago, the University of California Berkeley, Argonne National Laboratory, and Lawrence Berkeley National Laboratory has developed molecular qubits that bridge the gap between light and magnetism—and operate at the same frequencies as telecommunications technology. The advance, published today in Science, establishes a promising new building block for scalable quantum technologies that can integrate seamlessly with existing fiber-optic networks.
Because the new molecular qubits can interact at telecom-band frequencies, the work points toward future quantum networks—sometimes called the “quantum internet.” Such networks could enable ultra-secure communication channels, connect quantum computers across long distances, and distribute quantum sensors with unprecedented precision.
Molecular qubits could also serve as highly sensitive quantum sensors; their tiny size and chemical flexibility mean they could be embedded in unusual environments—such as biological systems —to measure magnetic fields, temperature, or pressure at the nanoscale. And because they are compatible with silicon photonics, these molecules could be integrated directly into chips, paving the way for compact quantum devices that could be used for computing, communication, or sensing.
A supercritical fluid refers to a state in which the temperature and pressure of a substance exceed its critical point, where no distinction exists between liquid and gas phases. Traditionally, it has been regarded as a single, uniform phase. However, a research team at POSTECH (Pohang University of Science and Technology) experimentally demonstrated nonequilibrium phase separation within supercritical fluids by observing nanometer-sized “liquid clusters” that persist for up to one hour.
The research team led by Professor Gunsu Yun from the Division of Advanced Nuclear Engineering and the Department of Physics at POSTECH, in collaboration with Dr. Jong Dae Jang’s group at the Korea Atomic Energy Research Institute (KAERI), Professor Min Young Ha at Kyung Hee University, and Dr. Changwoo Do’s team at Oak Ridge National Laboratory (ORNL) in the U.S., experimentally verified the existence of nano-clusters that exist separately in a liquid-like state within supercritical fluids previously considered a uniform phases.
The experiment utilized the Small-Angle Neutron Scattering (SANS) instrument at Korea’s neutron research facility, HANARO.
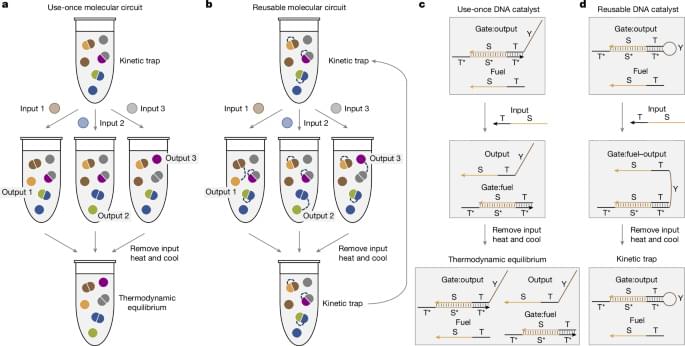
Though it might seem like science fiction, scientists are working to build nanoscale molecular machines that can be designed for myriad applications, such as “smart” medicines and materials. But like all machines, these tiny devices need a source of power, the way electronic appliances use electricity or living cells use ATP (adenosine triphosphate, the universal biological energy source).
Researchers in the laboratory of Lulu Qian, Caltech professor of bioengineering, are developing nanoscale machines made out of synthetic DNA, taking advantage of DNA’s unique chemical bonding properties to build circuits that can process signals much like miniature computers. Operating at billionth-of-a-meter scales, these molecular machines can be designed to form DNA robots that sort cargos or to function like a neural network that can learn to recognize handwritten numerical digits.
One major challenge, however, has remained: how to design and power them for multiple uses.