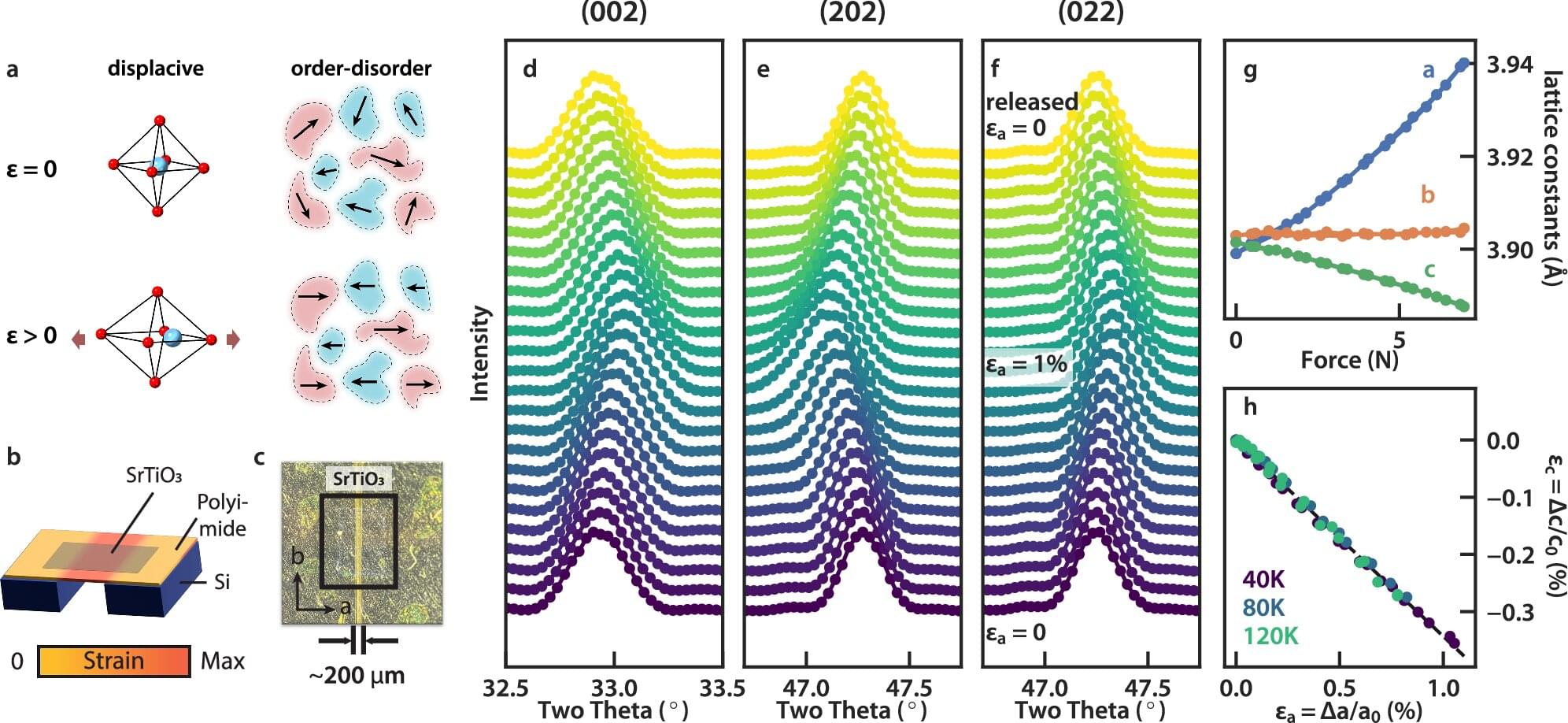
Strontium titanate was once used as a diamond substitute in jewelry before less fragile alternatives emerged in the 1970s. Now, researchers have explored some of its more unusual properties, which might someday be useful in quantum materials and microelectronics applications.
Writing in the journal Nature Communications, the team explains how they built an extremely thin, flexible strontium titanate membrane and stretched it, in the process turning on what’s known as a ferroelectric state. In that state, the material generates its own electric field, somewhat similar to how a permanent magnet generates its own magnetic field.
“We applied strain to tune the membrane to a ferroelectric or non-ferroelectric state reversibly and repeatedly,” said Wei-Sheng Lee, a lead scientist at the Department of Energy’s SLAC National Accelerator Laboratory and a principal investigator at the Stanford Institute for Materials and Energy Sciences (SIMES), a joint SLAC-Stanford institute. “This allowed quantitative characterizations of this transition in strontium titanate with unprecedented details.”