Click on photo to start video.
Ronald Kohanski, PhD. is the Deputy Director of the Division of Aging Biology at the National Institute on Aging, and he gave the keynote for day two of our recent conference in New York City.

The de Grey… AEWR.
Storytel, a monthly free trial: https://storytel.ru/bookperson
VK public page «Mustreads» where you can win the book «Ending Aging»: https://vk.com/mustreads
Mustreader: https://instagram.com/mustreader
Mustreads, Telegram: https://tlg.name/mustreads
Mustwatch, Telegram: https://tlg.name/mustwatch
Support the project on Patreon: https://patreon.com/mustreader
21 lessons for the 21st century by Yuval Noah Harari: https://amzn.to/2H6QN5u
Infinite Jest by David Foster Wallace: https://amzn.to/2Z8pHkq
The Fry Chronicles by Stephen Fry: https://amzn.to/2KAlLVR
SENS Research Foundation: https://www.sens.org/
Support SENS: https://www.sens.org/get-involved/
Podcast episode of Terminal Reading with KrioRus co-founder, part 1: https://tlg.name/mustreads/1981; part 2: https://tlg.name/mustreads/1989
Website of KrioRus: http://kriorus.ru/
Dr. Michael West, CEO of AgeX Therapeutics and Founder of Geron Corporation, discusses breakthroughs in the understanding of biological regeneration and in induced tissue regeneration, through his talk “Hayflick Rewound: Somatic Restriction, Epigenetics, and the Reversibility of Human Aging”. This talk was given at the Ending Age-Related Diseases conference in NYC. Join us at http://lifespan.io/hero
►Conference Page: https://www.leafscience.org/ending-age-related-diseases-adva…prospects/
►Subscribe for more: https://www.youtube.com/user/LifespanIO?sub_confirmation=1
►This video is presented by LEAF. Please support our work by becoming a “Lifespan Hero”: http://lifespan.io/hero
► #LifeExtension #MichaelWest #AgeX
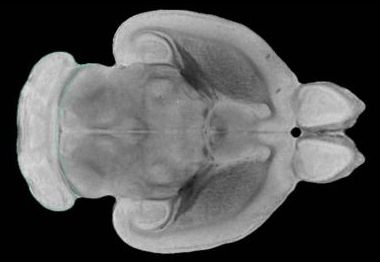
New research, published today in Nature, reveals how increasing brain stiffness as we age causes brain stem cell dysfunction, and demonstrates new ways to reverse older stem cells to a younger, healthier state.
The results have far reaching implications for how we understand the ageing process, and how we might develop much-needed treatments for age-related brain diseases.
As our bodies age, muscles and joints can become stiff, making everyday movements more difficult. This study shows the same is true in our brains, and that age-related brain stiffening has a significant impact on the function of brain stem cells.
Today, we’re releasing another keynote from Ending Age-Related Diseases 2019, our highly successful two-day conference that featured talks from leading researchers and investors, bringing them together to discuss the future of aging and rejuvenation biotechnology.
In his talk, Estimating the True Complexity of Comprehensive Rejuvenation, the famous Aubrey de Grey of SENS Research Foundation discussed the intricacies of creating a complete rejuvenation biotechnology framework, including the differing rates of age-related damage and the ramifications of the extensive crosstalk between different types of this damage.

A flamingo lives 40 years and a human being lives 90 years; a mouse lives two years and an elephant lives 60. Why? What determines the lifespan of a species? After analyzing nine species of mammals and birds, researchers at the Spanish National Cancer Research Center (CNIO) found a very clear relationship between the lifespan of these species and the shortening rate of their telomeres, the structures that protect the chromosomes and the genes they contain. The relationship is expressed as a mathematical equation, a formula that can accurately predict the longevity of the species. The study was done in collaboration with the Madrid Zoo Aquarium and the University of Barcelona.
“The telomere shortening rate is a powerful predictor of species lifespan,” the authors write in the prestigious journal Proceedings of the National Academy of Sciences (PNAS).
The study compares the telomeres of mice, goats, dolphins, gulls, reindeer, vultures, flamingos, elephants and humans, and reveals that species whose telomeres shorten faster have shorter lives.
Today, we want to highlight an interview with billionaire investor Jim Mellon that our friend, Adam Ford of Science, Technology, and the Future, has conducted. Like us, Adam was at the Undoing Aging conference in Berlin earlier this year, and, just as we were, he was busy conducting a number of interviews with the researchers and industry thought leaders there.
Jim Mellon is an interesting figure in the industry and the cofounder of Juvenescence, a company that has been investing in a number of promising companies that are developing rejuvenation biotechnology to treat age-related diseases.
In this video, he talks about how the company formed and the investment landscape in the aging research field, and he touches upon public availability of these technologies as well as overpopulation. Jim has been closely watching the industry for some years now, and he and his company have been making a number of investments in companies that are moving towards clinical trials.

With the rise of fad diets, “superfoods,” and a growing range of dietary supplement choices, it’s sometimes hard to know what to eat.
This can be particularly relevant as we grow older and are trying to make the best choices to minimize the risk of health problems such as high blood pressure, obesity, type 2 diabetes, and heart (cardiovascular) problems.
We now have evidence these health problems also all affect brain function: they increase nerve degeneration in the brain, leading to a higher risk of Alzheimer’s disease and other brain conditions including vascular dementia and Parkinson’s disease.