Oliver Zolman, best known for leading tech CEO Bryan Johnson’s anti-aging process, reportedly charges up to $1,000 per hour.
Category: life extension – Page 220
Retina cell breakthrough could help treat blindness
When the scaffold is treated with a steroid called fluocinolone acetonide, which protects against inflammation, the resilience of the cells appears to increase, promoting growth of eye cells. These findings are important in the future development of ocular tissue for transplantation into the patient’s eye.
Scientists have found a way to use nanotechnology to create a 3D ‘scaffold’ to grow cells from the retina-paving the way for potential new ways of treating a common cause of blindness.
Researchers, led by Professor Barbara Pierscionek from Anglia Ruskin University (ARU), have been working on a way to successfully grow retinal pigment epithelial (RPE) cells that stay healthy and viable for up to 150 days. RPE cells sit just outside the neural part of the retina and, when damaged, can cause vision to deteriorate.
It is the first time this technology, called ‘electrospinning’, has been used to create a scaffold on which the RPE cells could grow, and could revolutionise treatment for one of age-related macular degeneration, one of the world’s most common vision complaints.
Dr. Robert Sapolsky: Science of Stress, Testosterone & Free Will | Huberman Lab Podcast #35
In this episode, I interview Dr. Robert Sapolsky, Ph.D., Professor of Biology, Neurology & Neurosurgery at Stanford University. We discuss stress, what defines short-term versus long-term stress, and how stress can be beneficial or detrimental, depending on the context. We also discuss stress mitigation and how our sense of control over stress mitigation techniques, including exercise, determine health outcomes. Dr. Sapolsky explains some of the key effects of the hormone testosterone — how it can amplify pre-existing tendencies for aggression or sexual behavior, but that it does not produce those behaviors per se. He also explains how testosterone impacts our social hierarchies, sense of confidence, and willingness to embrace challenges of different kinds. He also explains how our behaviors and perceptions shape testosterone levels. And we discuss estrogen and the powerful role it plays in brain development, health and longevity. Finally, we discuss free will, what it means to have free will, and if we have any free will, including how knowledge alone might allow us to make better decisions for ourselves and society.
#HubermanLab #Testosterone #Stress.
Thank you to our sponsors:
ROKA — https://roka.com — use code “huberman“
InsideTracker — https://insidetracker.com/huberman.
Our Patreon page:
https://www.patreon.com/andrewhuberman.
Supplements from Thorne:
http://www.thorne.com/u/huberman.
Social:

Eight longevity habits that could lengthen your life by 20 years
Is there such a thing as a longevity mantra? A routine or set of guidelines that can help you extend your lifespan and healthspan – in a nutshell, giving you more life in your years and more years in your life?
A new study involving over 700,000 US veterans reports that people who adopt eight healthy lifestyle habits by middle age can expect to live substantially longer than those with few or none of these habits.
While it’s a list full of the usual suspects, having such a large data set has allowed the research team to put some numbers alongside these pillars of longevity.

Combining AI and MRI to improve human longevity
British health tech startup Twinn Health recently emerged from stealth, boasting an AI-powered platform that analyzes MRI scans to detect preventable disease “earlier than ever before.” Starting with metabolic disease, the company’s AI platform leverages validated imaging biomarkers to improve diagnosis and treatment decisions.
With age-related frailty and liver disease also on its roadmap, Twinn Health is positioning itself squarely in the domain of longevity and preventive healthcare. The company is supported by WAED, a $500 million venture capital fund backed by Saudi Aramco, which invests in innovative tech-based startups.
Longevity. Technology: Magnetic resonance imaging (MRI) has been used in healthcare for decades and is widely used in hospitals and clinics for the diagnosis and follow-up of disease. In recent years, AI tools have appeared that help identify the presence of specific conditions within MRI scans, but the technology is not yet widely used in healthcare to support healthspan and longevity improvements. Twinn Health aims to change that, combining MRI and AI to enable the early detection and management of multiple age-related diseases. To learn more, we caught up with founder and CEO Dr Wareed Alenaini.
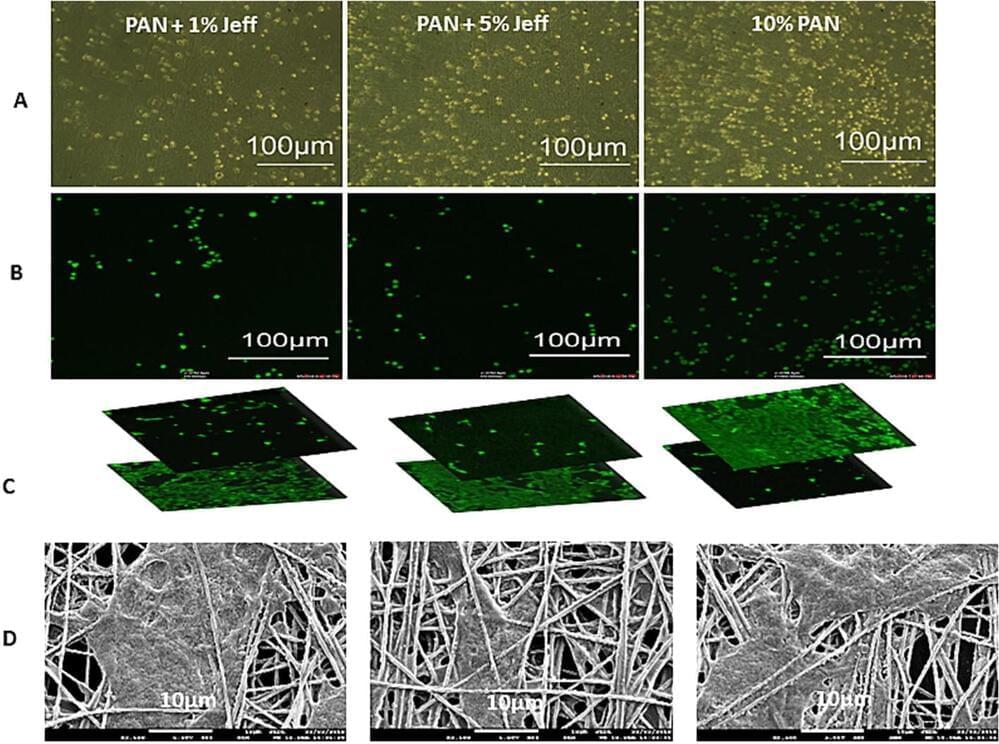
Retina cells cultured on nanofiber scaffolds could help treat blindness
Scientists have found a way to use nanotechnology to create a 3D “scaffold” to grow cells from the retina—paving the way for potential new ways of treating a common cause of blindness.
Researchers, led by Professor Barbara Pierscionek from Anglia Ruskin University (ARU), have been working on a way to successfully grow retinal pigment epithelial (RPE) cells that stay healthy and viable for up to 150 days. RPE cells sit just outside the neural part of the retina, and when damaged, can cause vision to deteriorate. Their work is published in Materials & Design.
It is the first time this technology, called “electrospinning,” has been used to create a scaffold on which the RPE cells could grow, and could revolutionize treatment for one of age-related macular degeneration, one of the world’s most common vision complaints.
Breakthrough in Age Reversal
Can the fountain of youth come in the form a pill?
Imagine this: a cocktail of specialized chemicals that rejuvenates your whole body, from your eyes and brain to your kidneys and muscles—bringing you back to a more youthful version of yourself.
Sound like science fiction?
The true costs of ageing
The rich world is ageing fast. How can societies afford the looming costs of caring for their growing elderly populations? film supported by @mission.winnow.
00:00 The wealthy world is ageing.
01:17 Japan’s elderly population.
02:11 The problems of an ageing world.
04:01 Reinventing old age.
05:48 Unlocking the potential of older years.
07:09 Reforming social care.
08:20 A community-based approach.
11:08 A fundamental shift is needed.
Read our special report on ageing and the economics of longevity here: https://econ.st/3EwnCV3
Sign up to The Economist’s daily newsletter to keep up to date with our latest stories: https://econ.st/3gJBH8D
Getting to grips with longevity: https://econ.st/3DBJU6k.
A small Japanese city shrinks with dignity: https://econ.st/3dBDgT2

