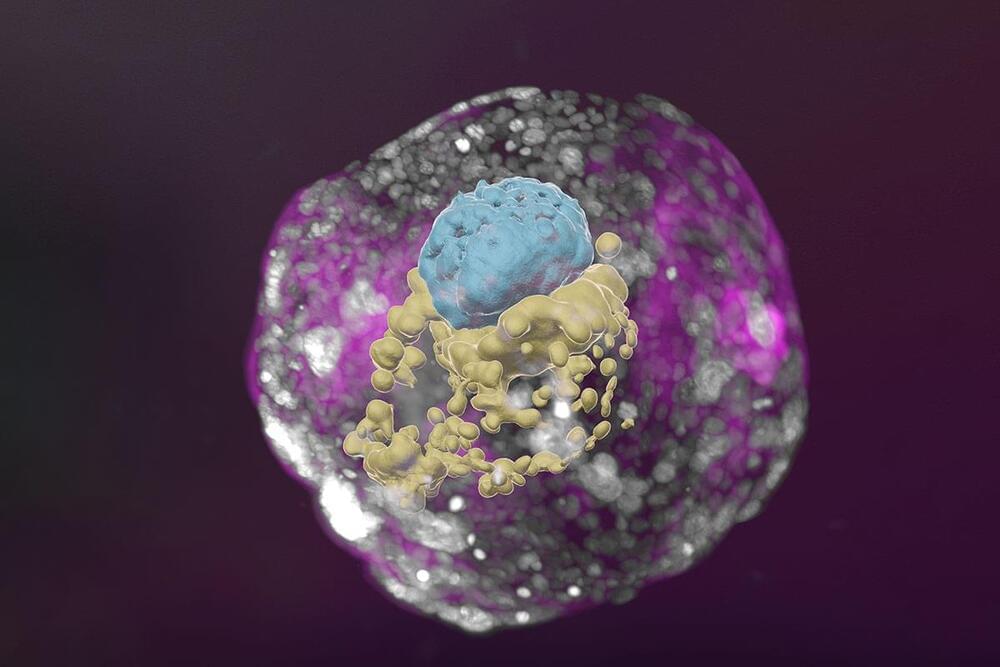
A research team headed by Professor Jacob Hanna at the Weizmann Institute of Science has created complete models of human embryos from stem cells cultured in the lab – and managed to grow them outside the uterus up to day 14. As reported today in Nature, these synthetic embryo models had all the structures and compartments characteristic of this stage, including the placenta, yolk sac, chorionic sac and other external tissues that ensure the models’ dynamic and adequate growth.
Longevity. Technology: Cellular aggregates derived from human stem cells in previous studies could not be considered genuinely accurate human embryo models, because they lacked nearly all the defining hallmarks of a post-implantation embryo. In particular, they failed to contain several cell types that are essential to the embryo’s development, such as those that form the placenta and the chorionic sac. In addition, they did not have the structural organization characteristic of the embryo and revealed no dynamic ability to progress to the next developmental stage.
Given their authentic complexity, the human embryo models obtained by Hanna’s group may not only provide an unprecedented opportunity to shed new light on the embryo’s mysterious beginnings, but open the door to new technologies for growing transplant tissues and organs.