Category: life extension – Page 194
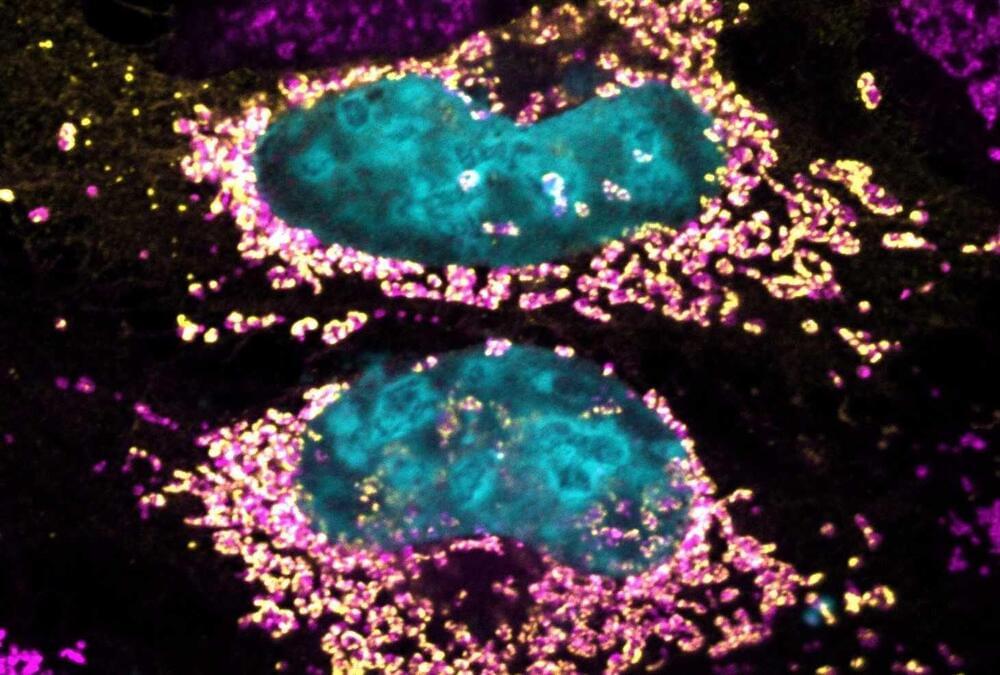
Study reveals bacterial protein capable of keeping human cells healthy
Researchers at the University of São Paulo (USP) in Brazil, partnering with colleagues in Australia, have identified a novel bacterial protein that can keep human cells healthy even when the cells have a heavy bacterial burden. The discovery could lead to new treatments for a wide array of diseases relating to mitochondrial dysfunction, such as cancer and auto-immune disorders. Mitochondria are organelles that supply most of the chemical energy needed to power cells’ biochemical reactions.
The study is published in the journal PNAS. The researchers analyzed more than 130 proteins released by Coxiella burnetii when this bacterium invades host cells, and found at least one to be capable of prolonging cell longevity by acting directly on mitochondria.
After invading host cells, C. burnetii releases a hitherto unknown protein, which the authors call mitochondrial coxiella effector F (MceF). MceF interacts with glutathione peroxidase 4 (GPX4), an anti-oxidant enzyme located in the mitochondria, to improve mitochondrial function by promoting an anti-oxidizing effect that averts cell damage and death, which may occur when pathogens replicate inside mammalian cells.

We all play don’t die every day — now let’s get really, really good at it
Bryan Johnson is the world’s most famous biohacker – and perhaps the “most measured man in human history”. He’s on a mission to maximally reverse the quantified biological age of each of his 70 organs, extending his lifespan and healthspan, and then roll out his protocol on a platform to ensure others can benefit from his experience, research and experimentation.
Johnson’s ethos can be summed up pretty neatly as don’t die, and to that end, he has written a book, or novel, to be more accurate, entitled Don’t Die, the uncorrected advanced reading version of which is downloadable as a free ebook from his website. Johnson’s nom de plume for this venture is Zero, described in the book as the “first individual H. sapiens to surpass five hundred years of age,” who dies in 2,478 (in an accident, rather than from old age), just weeks away from “becoming Homo Deus.” Johnson credits Zero with the invention of Zeroism and the resurrection technology undie, as well as the fathering of “millions of biological and digital offspring who now live in the far reaches of the solar system and beyond”
Longevity. Technology: Because Don’t Die is a novel, Johnson can explore his philosophy in a different way, inviting us to observe the narrator, Scribe, on his last day on Earth, as he muses on humanity’s future evolution, the nature of death and free will and the impact of age reversal and programmable biology. Scribe is joined by a Pilgrim’s Progress-like cast of characters, including Cognitive Bias, Dark Humor and Game Play and Self Critical.

Rejuvenation Startup Summit 2024 Announces First Speakers
The Rejuvenation Startup Summit (Berlin, May 10–11, 2024) hosted by the Forever Healthy Foundation, is a vibrant networking event that aims to accelerate the development of the rejuvenation biotech industry.
Rejuvenation/longevity biotech is a new, emerging field of medicine. It aims to prevent and reverse diseases of aging by addressing their common root cause, the aging process itself. Rejuvenation therapies aim to reverse or repair age-related cellular changes such as molecular waste, calcification, tissue stiffening, loss of stem cell function, genetic alterations, and impaired energy production.
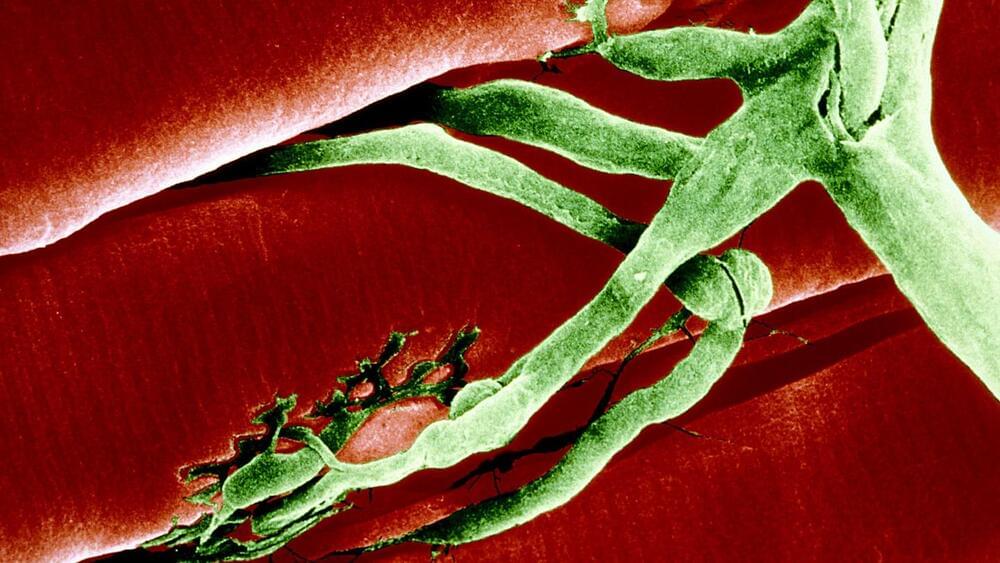
Blocking this one protein could strengthen muscles
Stanford researchers have finally figured out how a therapy that blocks a single protein can reverse age-related muscle loss in mice — and the discovery suggests seniors might not be the only ones who could benefit from it.
Age-related muscle loss: People tend to start losing muscle mass and strength in their 30s, and from 50 on, you could be losing up to 10% of your muscle mass every decade.
While it is possible to regain lost muscle through exercise, health issues can make hitting the weights a challenge. If left unaddressed, though, age-related muscle loss can lead to decreased mobility and weakness, which increases the risk of falls or other injuries.
The Kynurenine/Tryptophan Ratio: An Integrated Measure Of Many Pro- And Anti-Inflammatory Factors
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links:
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
NAD+ Quantification: https://www.jinfiniti.com/product/intracellular-nad-test/
Use Code: ConquerAging At Checkout.
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
Oral Microbiome: https://www.bristlehealth.com/?ref=michaellustgarten.
Enter Code: ConquerAging.
At-Home Blood Testing (SiPhox Health): https://getquantify.io/mlustgarten.
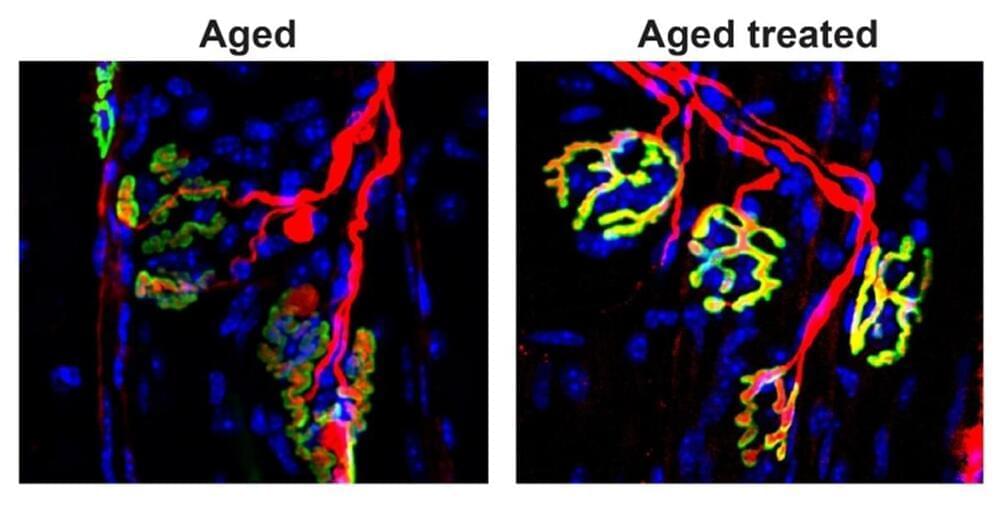
Blocking an aging-related enzyme may restore muscle strength
Stem cell biologist Helen Blau of Stanford University School of Medicine and colleagues previously found that blocking 15-PGDH in old mice restored their withered muscles and improved their strength after a month of treatment. On the flip side, young mice lost muscle and became weaker after their levels of this enzyme were increased for a month.
Blau’s team has now found that 15-PGDH accumulates in the muscles of old mice as the connections that allow communication between muscles and nerves are lost, another consequence of aging. Treating old mice for one month with a drug that inhibits 15-PGDH restored these connections, called synapses, between muscle fibers and motor nerve cells, and boosted the animals’ strength, the team reports in the Oct. 11 Science Translational Medicine. Those synapses are how the brain directs muscles to move.
The findings suggest that blocking the gerozyme 15-PGDH may be a way to help recover strength that has diminished due to nerve injuries, motor nerve cell diseases or aging.


15 Best Longevity Books for 2024
Looking for some good holiday reads? We’ve updated our list of best longevity books and added several from top longevity researchers like Dr. Harold Katcher, Dr. Morgan Levine, and Dr. Peter Attia.
Update 11/7/2023: This post has been updated since we originally published it in September 2020 and first updated it in April 2021. Several new best longevity books have been added to both the main list and honorable mentions sections, and the post has been cleaned up, and review ratings made current.
There are a lot of life extension and longevity books published every year.
A. Lot.

Researchers solve protein mystery
Researchers have uncovered that proteins use a common chemical label as a shield to protect them from degradation, which in turn affects motility and aging. Proteins are key to all processes in our cells and understanding their functions and regulation is of major importance.
“For many years, we have known that nearly all human proteins are modified by a specific chemical group, but its functional impact has remained undefined,” says professor Thomas Arnesen at the Department of Biomedicine, University of Bergen.