Dr. Sean Tucker, Ph.D. is the Founder and Chief Scientific Officer of Vaxart Inc. ( https://vaxart.com/ ), a clinical-stage biotechnology company developing…
Category: health – Page 66
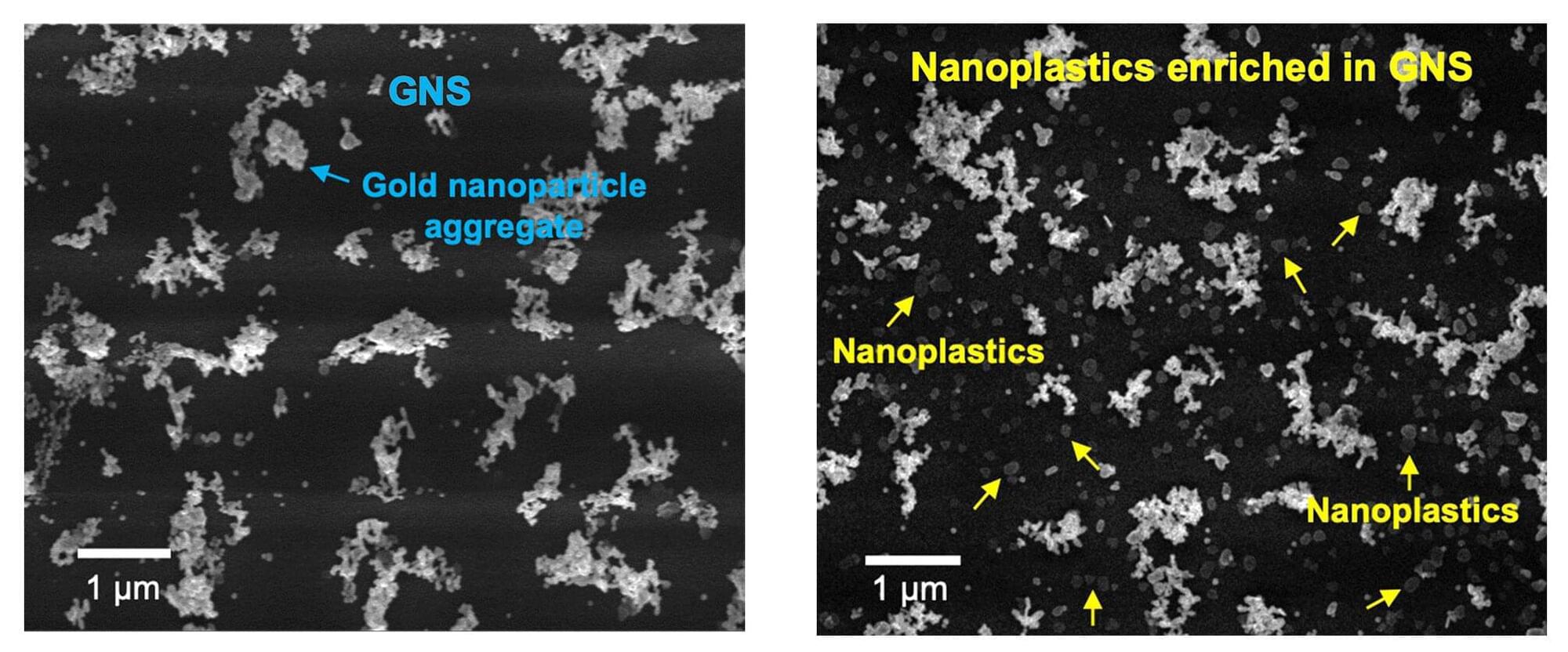
Scientists develop ultraprecise, efficient and flexible technique for counting and analyzing nanoplastics
While the threat that microplastics pose to human and ecological health has been richly documented and is well known, nanoplastics, which are smaller than one micrometer (1/50th the thickness of an average human hair), are far more reactive, far more mobile and vastly more capable of crossing biological membranes. Yet, because they are so tiny and so mobile, researchers don’t yet have an accurate understanding of just how toxic these particles are.
The first step to understanding the toxicology of nanoplastics is to build a reliable, efficient and flexible tool that can not only quantify their concentration in a given sample, but also analyze which specific plastics that sample contains.
An international team of scientists led by the University of Massachusetts Amherst reports in Nature Water on the development of a new tool, known as the OM-SERS setup, which can do all of these things and can furthermore be used to detect particular nanoplastic concentrations and polymer types in solid samples, such as soils, body tissues and plants.
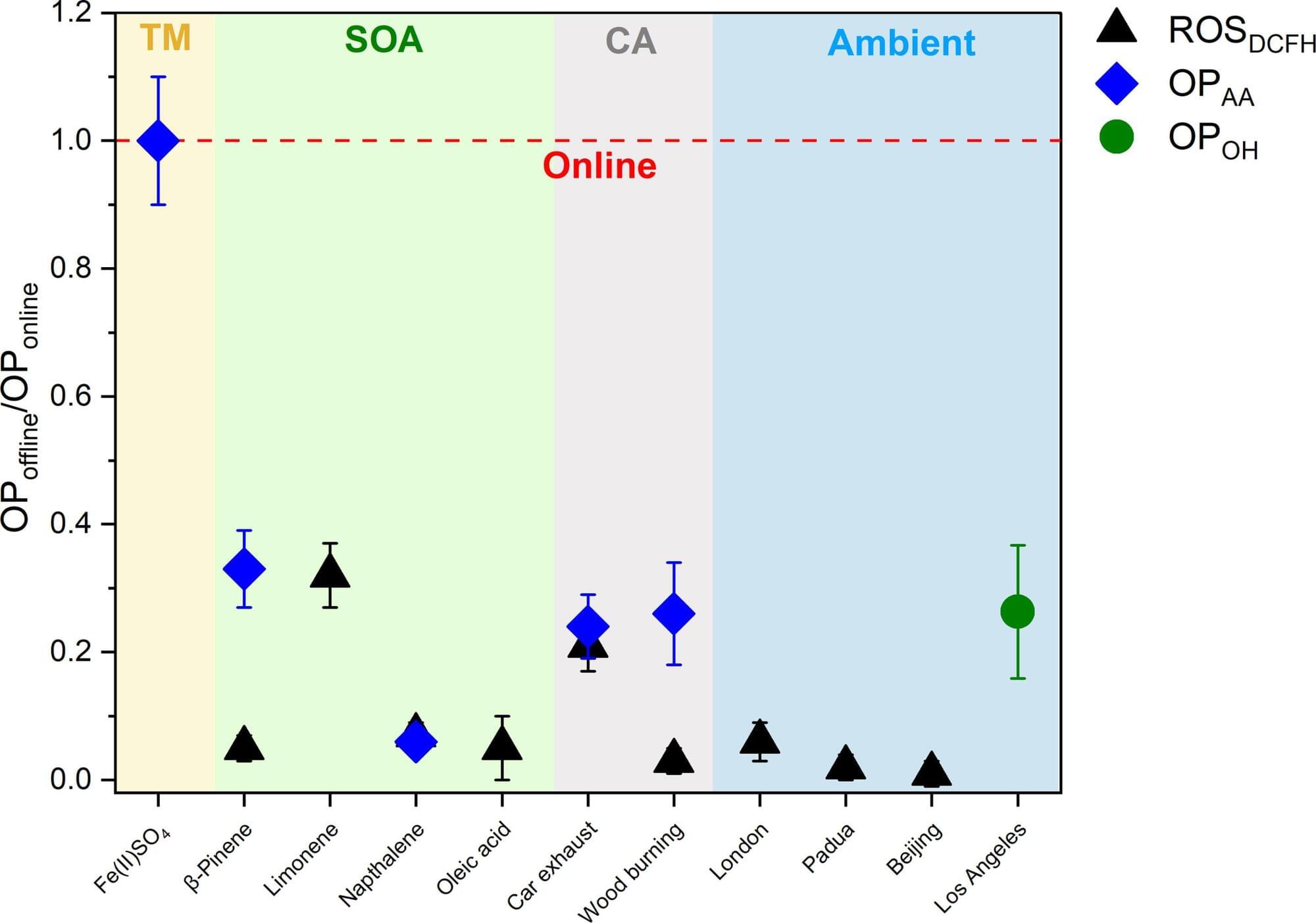
Real-time analysis reveals a much higher proportion of harmful substances in particulate matter than assumed
People breathing contaminated air over the course of years are at greater risk of developing numerous diseases. This is thought to be due to highly reactive components in particulate matter, which affect biological processes in the body. However, researchers from the University of Basel, Switzerland, have now shown that precisely these components disappear within hours and that previous measurements therefore completely underestimate the quantities in which they are present.
From chronic respiratory problems to cardiovascular diseases, diabetes and dementia, health damage caused by particulate matter air pollution is wide-ranging and serious. The World Health Organization (WHO) estimates that over six million deaths a year are caused by increased exposure to particulate matter.
The chemical composition of these tiny particles in the air, which come from a wide range of both anthropogenic and natural sources, is highly complex. Which particles trigger which reactions and long-term diseases in the body is the subject of intensive research.

SpaceX Falcon 9 Launch 🚀
SpaceX is set to launch Fram2 on Monday, March 31, at 9:46 p.m. ET from Launch Complex 39A at NASA’s Kennedy Space Center. If needed, additional opportunities extend through the early hours of April 1. This mission is unlike any before—it will take humanity to a polar orbit (90° inclination) for the first time! 🌍✨
🛰️ What Makes Fram2 Special?
🔥 First-ever human spaceflight to a true polar orbit.
👨🚀 All four astronauts—Wang, Mikkelsen, Rogge, and Philips—are first-time space travelers.
🩻 First medical X-ray taken in space.
🍄 Microgravity experiments, including mushroom cultivation.
💪 Independent crew exit post-splashdown—pushing the limits of astronaut endurance.
🚀 Falcon 9 and Dragon’s Role.
This mission will push the boundaries of Falcon 9 and Dragon’s ascent profile, showcasing the precision and power of SpaceX’s GNC (Guidance, Navigation, and Control) systems. After liftoff, the first stage booster will return to the droneship A Shortfall of Gravitas in the Atlantic Ocean. The Dragon capsule has a rich history, having previously flown on Crew-1, Inspiration4, and Polaris Dawn.
Fram2 is more than just a mission—it’s a bold step toward the future of space exploration. With 22 research experiments, including studies on human health in space, exercise physiology, and radiation exposure, this flight will pave the way for long-duration missions beyond Earth orbit.
Don’t miss this groundbreaking launch! Subscribe to Space Googlevesaire for real-time updates, expert breakdowns, and all things spaceflight! 🌌🚀🔔
Programmable pixels could advance infrared light applications
Without the ability to control infrared light waves, autonomous vehicles wouldn’t be able to quickly map their environment and keep “eyes” on the cars and pedestrians around them; augmented reality couldn’t display realistic 3D displays; doctors would lose an important tool for early cancer detection. Dynamic light control allows for upgrades to many existing systems, but complexities associated with fabricating programmable thermal devices hinder availability.
A new active metasurface, the electrically-programmable graphene field effect transistor (Gr-FET), from the labs of Sheng Shen and Xu Zhang in Carnegie Mellon University’s College of Engineering, enables the control of mid-infrared states across a wide range of wavelengths, directions, and polarizations. This enhanced control enables advancements in applications ranging from infrared camouflage to personalized health monitoring.
“For the first time, our active metasurface devices exhibited the monolithic integration of the rapidly modulated temperature, addressable pixelated imaging, and resonant infrared spectrum,” said Xiu Liu, postdoctoral associate in mechanical engineering and lead author of the paper published in Nature Communications. “This breakthrough will be of great interest to a wide range of infrared photonics, materials science, biophysics, and thermal engineering audiences.”

A breakthrough moment: Researchers discover new class of antibiotics
The last time a new class of antibiotics reached the market was nearly three decades ago—but that could soon change, thanks to a discovery by researchers at McMaster University.
A team led by researcher Gerry Wright has identified a strong candidate to challenge even some of the most drug-resistant bacteria on the planet: a new molecule called lariocidin. The findings were published in the journal Nature on March 26, 2025.
The discovery of the all-new class of antibiotics responds to a critical need for new antimicrobial medicines, as bacteria and other microorganisms evolve new ways to withstand existing drugs. This phenomenon is called antimicrobial resistance—or AMR—and it’s one of the top global public health threats, according to the World Health Organization.
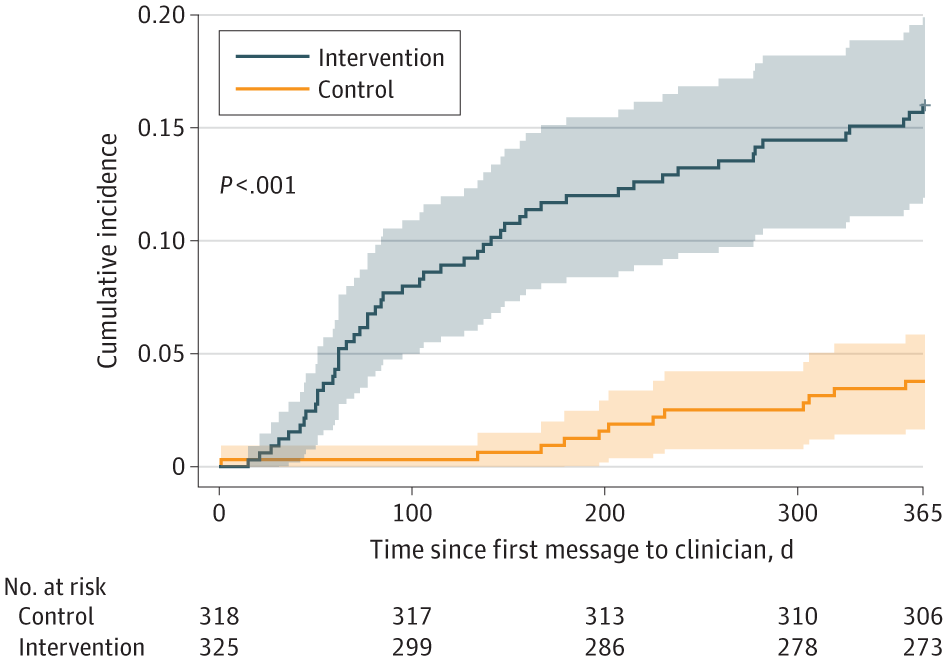
Leveraging Preexisting Cardiovascular Data to Improve the Detection and Treatment of Hypertension: The NOTIFY-LVH Randomized Clinical Trial
From JAMA Cardiol ogy: A centralized, population health coordinator-led notification and clinical support pathway improved the initiation of antihypertensive therapy in patients with left ventricular hypertrophy.
Despite the recognition that poorly controlled hypertension leads to adverse cardiovascular events, there are often barriers in care systems that contribute to substandard recognition and treatment.19 Notably, prior work employing trained nonphysicians focused on closing gaps in cardiovascular disease management has yielded significant improvements in disease-specific metrics using remote, centralized interventions.20-25 Similarly, there is a growing body of evidence demonstrating the effectiveness of clinician-directed support systems—often in the form of “nudges”—that have made meaningful advances in a variety of clinical outcomes.26,27 Whether a methodologic approach combining clinician nudges with the support of trained nonphysicians can be applied to LVH-associated diseases—including hypertension—is unknown.
Accordingly, the NOTIFY-LVH pragmatic randomized clinical trial28 sought to determine whether potentially underutilized echocardiogram data could be leveraged to improve patient care by augmenting the traditional ambulatory care framework. Specifically, this study tested whether a centralized clinical support pathway targeting clinicians of patients with LVH on their prior echocardiograms would increase the rate of treatment for hypertension and the earlier diagnosis of LVH-associated diseases.
Dangerous Fungal Infection Sees a Dramatic Increase in US Hospitals
Cases of the fungal infection Candida auris are rising rapidly and coming from more sources too, a new US study reveals.
C. auris was first reported in the US in 2016 and is considered an “urgent antimicrobial resistance threat” in hospitals, according to the Centers for Disease Control and Prevention (CDC).
Focusing on a large health system in Miami, Florida, the new research found that reported clinical cases had risen from 5 in 2019 to 115 in 2023 – a considerable jump of 2,200 percent in four years.

NASA continues BioNutrients space-fermented food research
NASA’s BioNutrients series of experiments is testing ways to use microorganisms to make nutrients that will be needed for human health during future long-duration deep space exploration missions.
Some vital nutrients lack the shelf-life needed to span multi-year human missions, such as a mission to Mars, and may need to be produced in space to support astronaut health. To meet this need, the BioNutrients project uses a biomanufacturing approach similar to making familiar fermented foods, such as yogurt. But these foods will also include specific types and amounts of nutrients that crews will be able to consume in the future.
The first experiment in the series, BioNutrients-1, set out to assess the five-year stability and performance of a hand-held system—called a production pack—that uses an engineered microorganism, yeast, to manufacture fresh vitamins on-demand and in space.