Category: genetics – Page 505
CRISPR has been used to treat US cancer patients for the first time
The gene-editing tool has been used in a trial to enhance the blood cells of two patients with cancer.
The trial: The experimental research, under way at the University of Pennsylvania, involves genetically altering a person’s T cells so that they attack and destroy cancer. A university spokesman confirmed it has treated the first patients, one with sarcoma and one with multiple myeloma.
Slow start: Plans for the pioneering study were first reported in 2016, but it was slow to get started. Chinese hospitals, meanwhile, have launched a score of similar efforts. Carl June, the famed University of Pennsylvania cancer doctor, has compared the Chinese lead in employing CRISPR to a genetic Sputnik.
Discovery of oral cancer biomarkers could save thousands of lives
Oral cancer is known for its high mortality rate in developing countries, but an international team of scientists hope its latest discovery will change that.
Researchers from the University of Otago and the Indian Statistical Institute (ISI), Kolkata, have discovered epigenetic markers that are distinctly different in oral cancer tissues compared to the adjacent healthy tissues in patients.
Co-author Dr. Aniruddha Chatterjee, of Otago’s Department of Pathology, says finding these biomarkers is strongly associated with patient survival.
Scientists reveal connection between cancer and human evolution
Researchers at Ben-Gurion University of the Negev (BGU) have discovered that gene mutations that once helped humans survive may increase the possibility for diseases, including cancer.
The findings were recently the cover story in the journal Genome Research.
The team of researchers from BGU’s National Institute for Biotechnology in the Negev (NIBN) set out to look for mutations in the genome of the mitochondria, a part of every cell responsible for energy production that is passed exclusively from mothers to their children. The mitochondria are essential to every cell’s survival and our ability to perform the functions of living.
How to biohack your cells to fight cancer — Greg Foot
Check out the science of biohacking, where biologists go into a patient’s genetic code and reprogram their immune system to recognize and fight cancer cells.
-
The human body is made up of about 30 trillion cells that carry a code which has been duplicated over and over for billions of years — with varying degrees of accuracy. So what happens when the system breaks down and the machinery turns on itself, leading to cancer? Greg Foot dives into the science of how biologists are biohacking the human body to try to fix the seemingly unfixable.
Lesson by Greg Foot, directed by Pierangelo Pirak.
Produced for ted-ed by NIHR university college london hospitals biomedical research centre.
Sign up for our newsletter: http://bit.ly/TEDEdNewsletter
What Interesting Trends Are We Seeing In Genetics Research Right Now?
What interesting trends are we seeing in genetics research right now? originally appeared on Quora: the place to gain and share knowledge, empowering people to learn from others and better understand the world.
Answer by Carrie Northover, Research Director at 23andMe, on Quora:
One of the coolest things right now is the size and scale of the research we’re able to do with human genetics, and those numbers are just getting bigger and bigger.
Shutting down deadly pediatric brain cancer at its earliest moments
Cell-by-cell genetic analyses of developing brain tissues in neonatal mice and laboratory models of brain cancer allowed scientists to discover a molecular driver of the highly aggressive, deadly, and treatment-resistant brain cancer, glioblastoma.
Published findings in Cell Stem Cell describe how the single-cell analyses identified a subpopulation of cells critical to glioblastoma formation—the early primitive progenitor cells of oligodendrocyte brain cells, pri-OPC progenitors, according to Q. Richard Lu, Ph.D., lead investigator and Scientific Director of the Brain Tumor Center at Cincinnati Children’s Hospital Medical Center.
The data suggest that reprogramming of primitive oligodendrocyte progenitors into a stem-like state plays an important role in glioma initiation and progression. The researchers’ primary molecular target in the study, a protein called Zfp36l1, launches biological programs that mirror those of healthy early brain development in the mice, but instead help fuel brain cancer growth. The discovery presents an opportunity to find out if new therapeutic approaches can stop glioblastoma at its earliest stages of initial formation or recurrence, Lu said.
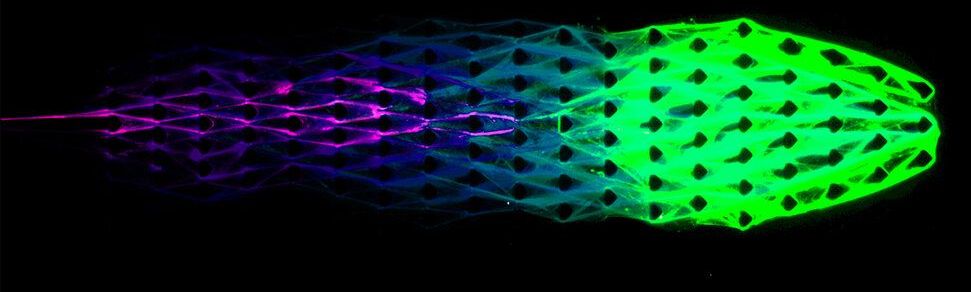
Engineers tap DNA to create ‘lifelike’ machines
As a genetic material, DNA is responsible for all known life. But DNA is also a polymer. Tapping into the unique nature of the molecule, Cornell engineers have created simple machines constructed of biomaterials with properties of living things.
Using what they call DASH (DNA-based Assembly and Synthesis of Hierarchical) materials, Cornell engineers constructed a DNA material with capabilities of metabolism, in addition to self-assembly and organization – three key traits of life.
“We are introducing a brand-new, lifelike material concept powered by its very own artificial metabolism. We are not making something that’s alive, but we are creating materials that are much more lifelike than have ever been seen before,” said Dan Luo, professor of biological and environmental engineering in the College of Agriculture and Life Sciences.