Scientists successfully treated a rare disease with the experimental gene-editing technique. It could open the door to new ways of treating more common disorders in the future.



Move over, gene-editing proteins—there’s a smaller, cheaper, more specific genetic engineering tool on the block: DNAzymes—small DNA molecules that can function like protein enzymes.
Researchers at the University of Illinois Urbana-Champaign have developed a technique that, for the first time, allows DNAzymes to target and cut double-stranded DNA, overcoming a significant limitation of the technology. DNAzymes have been used in biosensing, DNA computing and many other applications. However, when it comes to genetic engineering applications such as gene editing or gene therapy, they have faced a challenge: DNAzymes have only been able to target sites on single-stranded DNA, while the DNA coding for genes in cells is double-stranded. The researchers published their new technique in the Journal of the American Chemical Society.
“DNAzymes have many advantages, including higher stability, smaller size and lower cost than protein enzymes. These advantages perfectly fit the requirement for genetic engineering tools,” said study leader Yi Lu, a professor of chemistry at Illinois. “No DNAzymes could alter double-stranded DNA until this work. By making that happen, we open the door for DNAzymes to enter the entire world of genetic engineering.”

To understand how the clones can create millions of copies of themselves and yet remain functional, Oldroyd and his team compared the genomes of Cape honeybee workers with those of their queen and her offspring.
After forcing the Cape queen to reproduce asexually by fitting her with surgical tape that prevented her from mating, the team examined certain DNA sequences of both the Cape queen and the 25 larvae she produced. Then, they did the same for four Cape honeybee workers and their 63 larvae.
The team discovered that the asexually reproduced offspring of the queen had levels of recombination (DNA mixing) 100 times greater than the genetically identical cloned offspring of the workers — a finding that suggests the Cape worker bees have evolved a mutation that prevents recombination. Without the risk of a one-third loss of genetic material caused by the asexual reshuffling process, the workers are free to continually create perfect copies of themselves.
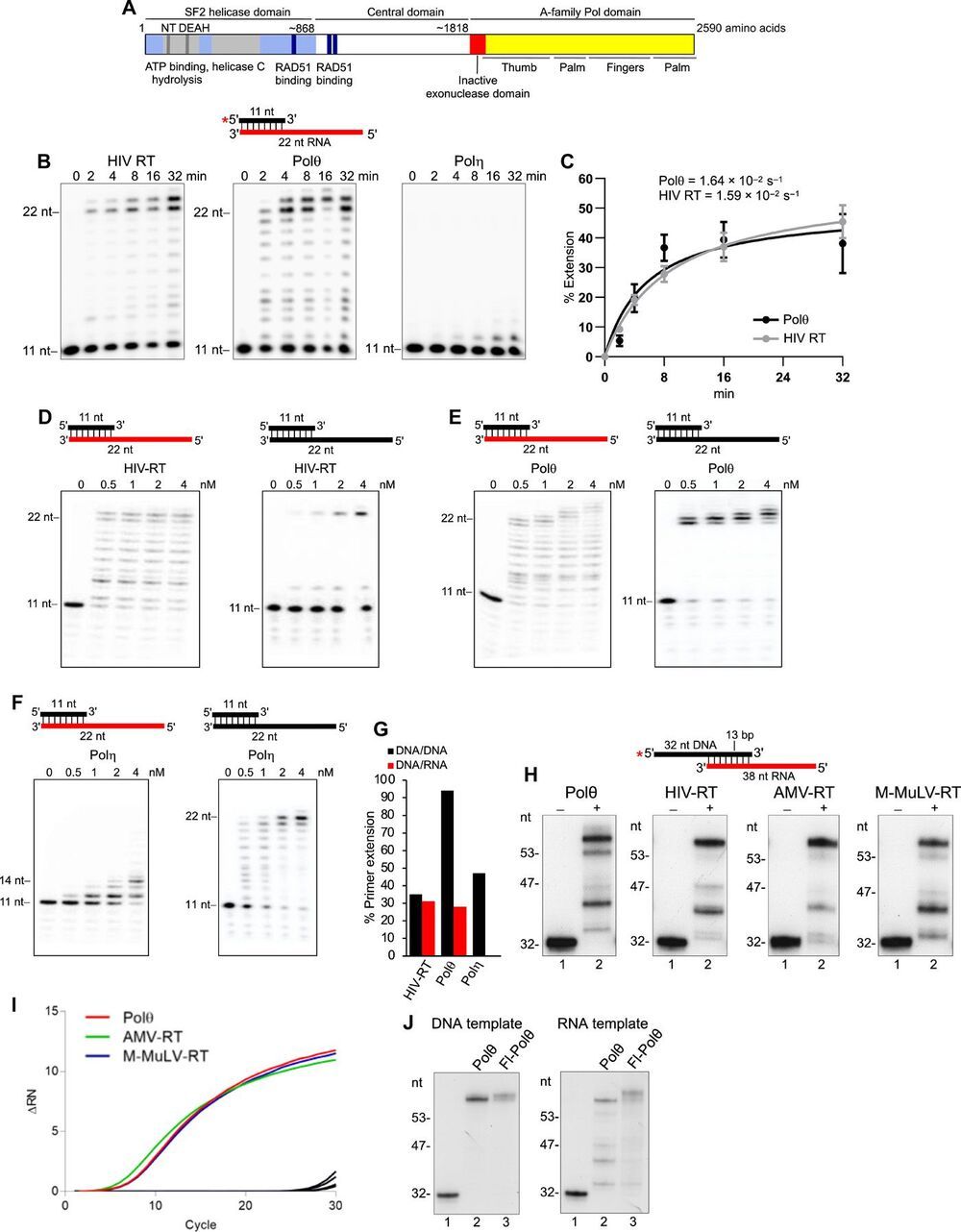
Genome-embedded ribonucleotides arrest replicative DNA polymerases (Pols) and cause DNA breaks. Whether mammalian DNA repair Pols efficiently use template ribonucleotides and promote RNA-templated DNA repair synthesis remains unknown. We find that human Polθ reverse transcribes RNA, similar to retroviral reverse transcriptases (RTs). Polθ exhibits a significantly higher velocity and fidelity of deoxyribonucleotide incorporation on RNA versus DNA. The 3.2-Å crystal structure of Polθ on a DNA/RNA primer-template with bound deoxyribonucleotide reveals that the enzyme undergoes a major structural transformation within the thumb subdomain to accommodate A-form DNA/RNA and forms multiple hydrogen bonds with template ribose 2′-hydroxyl groups like retroviral RTs. Last, we find that Polθ promotes RNA-templated DNA repair in mammalian cells. These findings suggest that Polθ was selected to accommodate template ribonucleotides during DNA repair.
Polymerase θ (Polθ) is a unique DNA polymerase-helicase fusion protein in higher eukaryotes whose A-family polymerase domain evolved from Pol I enzymes (Fig. 1A) (1, 2). However, contrary to most Pol I enzymes, Polθ is highly error-prone and promiscuous (3–6), performs translesion synthesis (TLS) opposite DNA lesions (3, 7, 8), and facilitates microhomology-mediated end-joining (MMEJ) of double-strand breaks (DSBs) by extending partially base-paired 3′ single-stranded DNA (ssDNA) overhangs at DSB repair junctions (5, 9–12). Polθ is not expressed in most tissues but is highly expressed in many cancer cells, which corresponds to a poor clinical outcome (13, 14). Furthermore, Polθ confers resistance to genotoxic cancer therapies and promotes the survival of cells deficient in DNA damage response pathways (11, 13–16). Thus, Polθ represents a promising cancer drug target.
Intriguingly, Polθ has an inactive proofreading domain due to acquired mutations (Fig. 1A) (2). Inactivating the 3′-5′ proofreading function of closely related A-family bacterial Pol I Klenow fragment (KF) enables this polymerase to reverse transcribe RNA like retroviral reverse transcriptases (RTs), which lack proofreading activity (fig. S1A) (17, 18). Because Polθ is highly error-prone and promiscuous and contains an inactive proofreading domain, we hypothesized that it has RNA-dependent DNA synthesis activity. Given that ribonucleotides are the most frequently occurring nucleotide lesion in genomic DNA that arrest replicative Pols and cause DNA breaks (19, 20), we also envisaged that Polθ would tolerate template ribonucleotides during its DNA repair activities and thus promote RNA-templated DNA repair synthesis (RNA-DNA repair). Although RNA-DNA repair mechanisms have been demonstrated in genetically engineered yeast cells (21, 22), they remain obscure in mammalian cells.

STANFORD, Calif. — A groundbreaking “superhero” vaccine inspired by the DNA code of Olympic athletes could help transform society over the next decade, a top genetic scientist claims.
The vaccine would provide lifelong protection against three of the top ten leading causes of death, according to Euan Ashley, professor of medicine and genetics at Stanford University. The so-called “superhero” jab could offer simultaneous, long-term protection against heart disease, stroke, Alzheimer’s disease, and liver disease, thanks to advances in genetic engineering.
This breakthrough treatment would deliver the blueprint of “ideal” cells from men and women whose genes are more disease-resistant than those of the average person, together with an “instruction manual” to help the body “repair, tweak and improve” its own versions. A single dose could lead to a “body-wide genetic upgrade” that would cut the risk of premature death in some adults by as much as 50 percent.
“At first, we had a hard time believing the results. Many of these genes are classical hallmarks of aging and yet our results suggested that their activity is more a function of the presence of bacteria rather than the aging process,” said Dr. Shukla.
Notably, this included genes that control stress and immunity. The researchers tested the impact that the antibiotics had on these genes by starving some flies or infecting others with harmful bacteria and found no clear trend. At some ages, the antibiotics helped flies survive starvation or infection longer than normal whereas at other ages the drugs either had no effect or reduced the chances of survival.
NIH scientists discover that bacteria may drive activity of many hallmark aging genes in flies.
To better understand the role of bacteria in health and disease, National Institutes of Health researchers fed fruit flies antibiotics and monitored the lifetime activity of hundreds of genes that scientists have traditionally thought control aging. To their surprise, the antibiotics not only extended the lives of the flies but also dramatically changed the activity of many of these genes. Their results suggested that only about 30% of the genes traditionally associated with aging set an animal’s internal clock while the rest reflect the body’s response to bacteria.
“For decades scientists have been developing a hit list of common aging genes. These genes are thought to control the aging process throughout the animal kingdom, from worms to mice to humans,” said Edward Giniger, Ph.D., senior investigator, at the NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and the senior author of the study published in iScience. “We were shocked to find that only about 30% of these genes may be directly involved in the aging process. We hope that these results will help medical researchers better understand the forces that underlie several age-related disorders.”
The results happened by accident. Dr. Giniger’s team studies the genetics of aging in a type of fruit fly called Drosophila. Previously, the team showed how a hyperactive immune system may play a critical role in the neural damage that underlies several aging brain disorders. However, that study did not examine the role that bacteria may have in this process.

The scientists found “biotin paint” on a protein named RSK1, which is part of a complex that keeps a nearby group of proteins, called RAS proteins, dormant. The scientists were surprised to discover that when they inactivated mutant KRAS, the nearby RSK1 complex stopped working as well. This allowed the RAS proteins to activate and take over the work of the missing mutant KRAS.
Cancer cells can become resistant to treatments through adaptation, making them notoriously tricky to defeat and highly lethal. Cold Spring Harbor Laboratory (CSHL) Cancer Center Director David Tuveson and his team investigated the basis of “adaptive resistance” common to pancreatic cancer. They discovered one of the backups to which these cells switch when confronted with cancer-killing drugs.
KRAS is a gene that drives cell division. Most pancreatic cancers have a mutation in the KRAS protein, causing uncontrolled growth. But, drugs that shut off mutant KRAS do not stop the proliferation. The cancer cells find a way to bypass the blockage and keep on dividing. Derek Cheng, the lead author of the study and a former Medical Scientist Training Program student in the Tuveson lab, compares this process to backup engines on a ship. He says, “You take away your main engine, you’re kind of on some backup engines. But it’s getting by on those. The ship isn’t sinking yet. It’s still moving at a slower pace. Ultimately what we want to do is sink the ship.”
Tuveson and his team wanted to figure out the “backup engines” in these cancer cells. They used a technique called biotin proximity labeling to identify what other proteins interacted with mutant KRAS. Cheng says, “I basically attach a spray can to my favorite protein, or rather least favorite protein, in this case. And so it attaches biotin, basically spraying biotin ‘paint’ to nearby proteins, and we’re able to analyze it to figure out what proteins were labeled.”

😀
Cellular senescence, a state of permanent growth arrest, has emerged as a hallmark and fundamental driver of organismal aging. It is regulated by both genetic and epigenetic factors. Despite a few previously reported aging-associated genes, the identity and roles of additional genes involved in the regulation of human cellular aging remain to be elucidated. Yet, there is a lack of systematic investigation on the intervention of these genes to treat aging and aging-related diseases.
How many aging-promoting genes are there in the human genome? What are the molecular mechanisms by which these genes regulate aging? Can gene therapy alleviate individual aging? Recently, researchers from the Chinese Academy of Sciences have shed new light on the regulation of aging.
Recently, researchers from the Institute of Zoology of the Chinese Academy of Sciences (CAS), Peking University, and Beijing Institute of Genomics of CAS have collaborated to identify new human senescence-promoting genes by using a genome-wide CRISPR/Cas9 screening system and provide a new therapeutic approach for treating aging and aging-related pathologies.
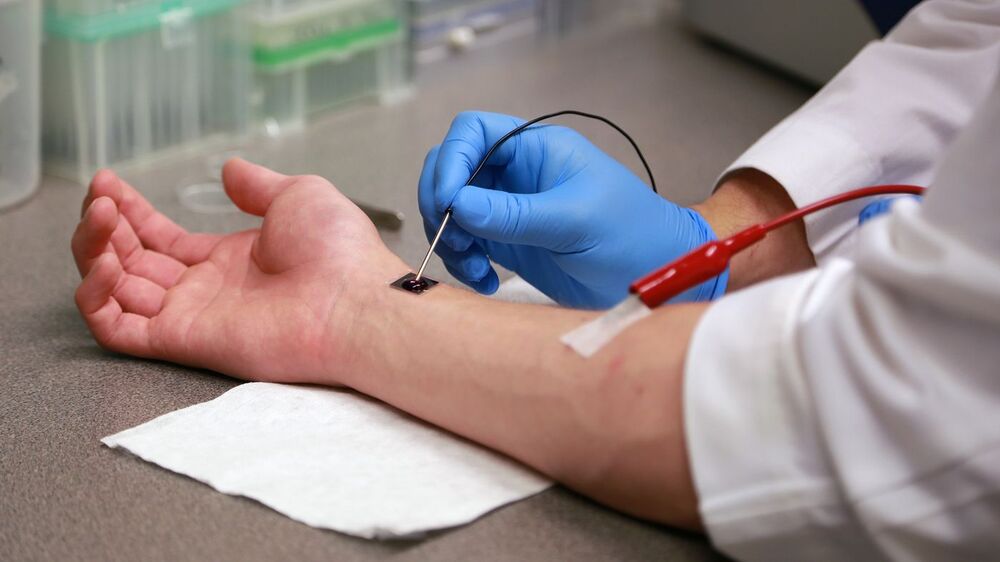
Circa 2017 using this can lead to near Ironman or foglet bodies with the ability to self heal the human body. It could be used on smartphones to heal people not needing a doctor in the future. This also would allow for the biological singularity to happen.
This device shoots new genetic code into cells to make them change their purpose. Researchers say the chip could someday be used to treat injuries in humans. But they’ve got a long, long way to go.