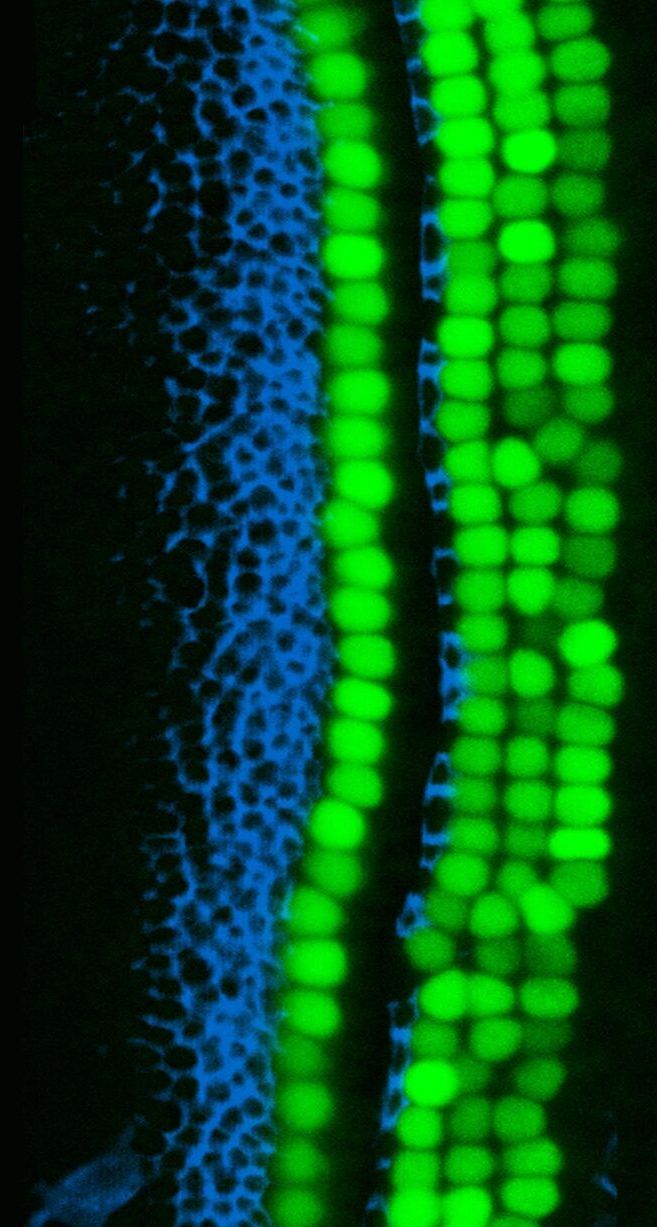
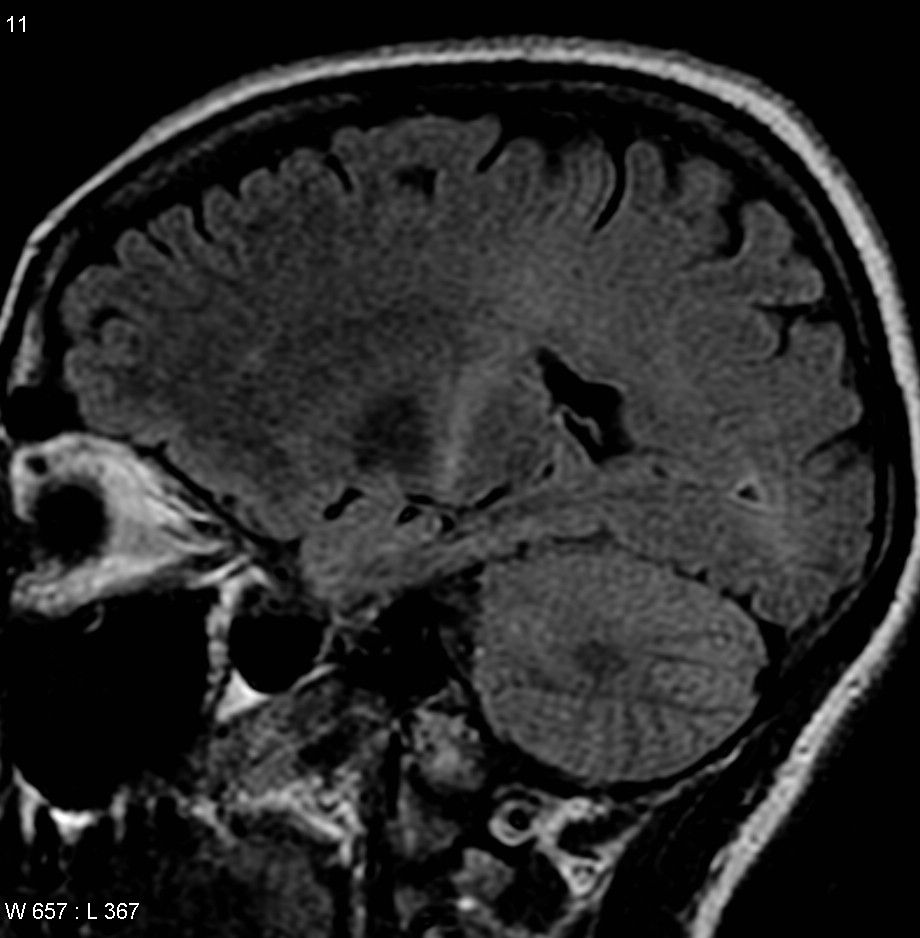
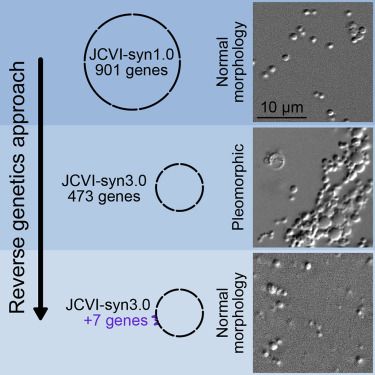
“Balancing that, I clearly state that my goal is not longevity, not even modest longevity. It’s just reversal of diseases of aging, which really is classic medicine. Q: Which takes me to the next question: do we even know how to aim at life extension? I don’t think we do. I think if we get serious aging reversal, it’s something that we can continue to improve on, just like we improved on transportation from the first wheel to rocket ships,” I’ll be honest, I disagree as we have some improvement in humans indicated from TRIM and TAME and plasma filtering. Church’s work is very important though.
Professor of Genetics at Harvard Medical School and one of the most prominent geroscientists, George Church works on gene therapies that can potentially reverse age-related diseases. We had the opportunity to interview this prolific researcher and entrepreneur, who is involved in dozens of startups on topics ranging from the current state of gene therapy to his recent attempt to auction off his genome, one of the first sequenced human genomes in the world, as an NFT.
What have been the successes and the failures of gene therapy in recent years? What do you expect to happen in the next few years?
So, most of the big failures of gene therapy happened at the very beginning, around the year 2000, almost two decades ago, when a couple of people died from an LMO2 oncogene, and one person died from an immune reaction to an adenovirus vector. So, that was 20 years ago. Fast forward to now, and gene therapies are mostly succeeding, hundreds of them are in clinical trials, you have dozens that have been approved by the FDA.