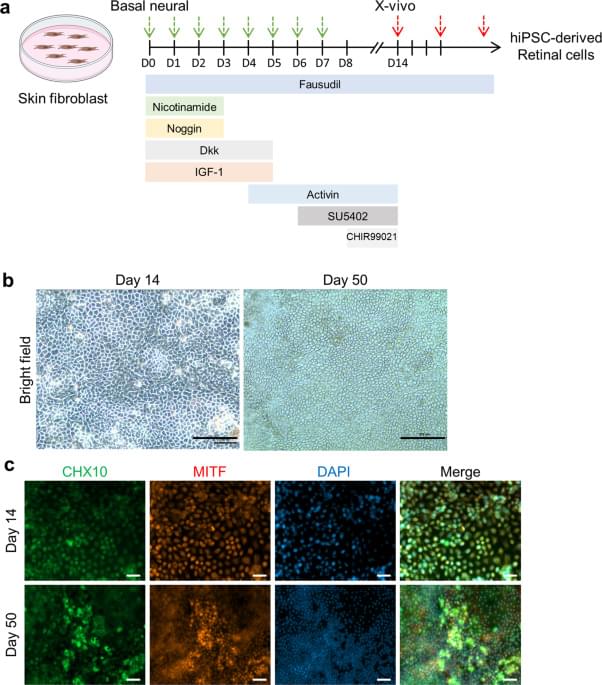
Circa 2021 First breakthrough in immortality of the eyes of rats using the inducing of pluripotent stem cells in the eye. Which will eventually lead to immortality of the human eye.
The retina is neural tissue located in the posterior part of the eye and is an extension of the central nervous system (CNS), which has limited regenerative potential once damaged1. Therefore, to maintain homeostasis of the retinal microenvironment and protect itself from harmful stimuli, the retina has a unique structure consisting of inner and outer blood-retinal barriers (BRBs)2,3,4. The outer BRB is mainly composed of retinal pigment epithelial (RPE) cells, which support photoreceptor cells, the primary neurons in the retina, and play a significant role in the pathogenesis of retinal degenerative disorders, such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP)5,6,7,8,9. These disorders are commonly characterized by the irreversible loss of photoreceptor cells and RPE cells, and the only fundamental treatment for these retinal degenerative disorders is replacement of damaged or atrophied cells10,11,12. Thus, regenerative treatments, such as stem cell transplantation, are emerging as attractive options for targeting retinal degeneration that was previously considered untreatable13.
RP refers to a set of hereditary retinal degenerative disorders that initially involve photoreceptors and leads to subsequent RPE cell damage; it affects 1 in 4,000 individuals worldwide9. Due to its inherent nature, extensive genetic studies are ongoing, and more than 50 causal genes have been identified14. Among the causal genes, PDE6B is a gene that encodes rod cGMP-phosphodiesterase, which is a critical component of the biochemical light transduction pathway9. Although various molecular and genetic studies have identified the pathomechanisms of RP, attempts to restore vision in patients with RP have failed. To overcome this issue, preclinical stem cell-based studies involving transient dosing or permanent implantation of pluripotent stem cells are being conducted10,11,15,16.
Permanent implantation of retinal stem cells is a promising method and is highly expected to be a potential alternative treatment strategy for replacing damaged retinal cells13,16. Sharma et al.17 manufactured clinical-grade AMD patient stem cell-derived RPE cells using RPE patches of a biodegradable scaffold, and functionally validated the effects of their transplantation. This researchers provided a pipeline for the generation of clinical-grade induced pluripotent stem cell (iPSC)-derived RPE cells, and histologically and functionally validated the efficacy of transplantation, thereby significantly advancing the retinal stem cell transplantation field; however, a single RPE cell transplantation cannot rescue already compromised photoreceptor cells and can be only applied in early stages of retinal degenerative diseases, when there are sufficient functional photoreceptor cells.