Using lipid nanoparticles (LNPs), engineers have successfully delivered genetic material to the lung that suppresses lung tumors in mice.
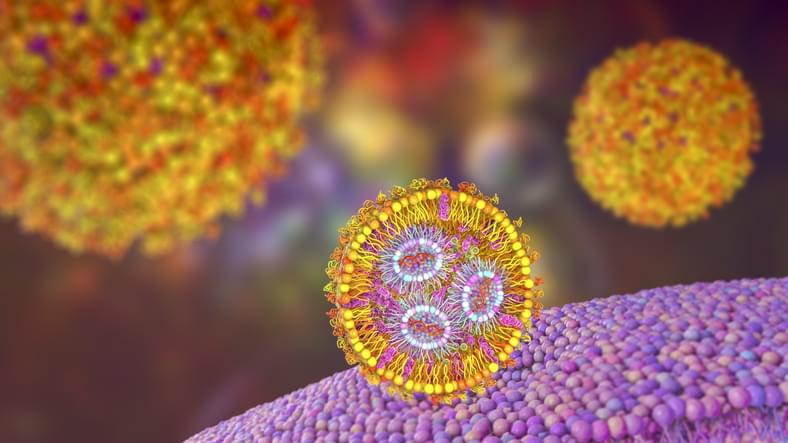

A computational model of the more than 26 million atoms in a DNA-packed viral capsid expands our understanding of virus structure and DNA dynamics, insights that could provide new research avenues and drug targets, University of Illinois Urbana-Champaign researchers report in the journal Nature.
“To fight a virus, we want to know everything there is to know about it. We know what’s inside in terms of components, but we don’t know how they’re arranged,” said study leader Aleksei Aksimentiev, an Illinois professor of physics. “Knowledge of the internal structures gives us more targets for drugs, which tend to focus on receptors on the surface or replication proteins.”
Viruses keep their genetic material —either DNA or RNA—packaged in a hollow particle called a capsid. While the structures of many hollow capsids have been described, the structure of a full capsid and the genetic material inside it has remained elusive.

At the end of the 20th century, analog systems in computer science have been widely replaced by digital systems due to their higher computing power. Nevertheless, the question keeps being intriguing until now: is the brain analog or digital? Initially, the latter has been favored, considering it as a Turing machine that works like a digital computer. However, more recently, digital and analog processes have been combined to implant human behavior in robots, endowing them with artificial intelligence (AI). Therefore, we think it is timely to compare mathematical models with the biology of computation in the brain. To this end, digital and analog processes clearly identified in cellular and molecular interactions in the Central Nervous System are highlighted. But above that, we try to pinpoint reasons distinguishing in silico computation from salient features of biological computation. First, genuinely analog information processing has been observed in electrical synapses and through gap junctions, the latter both in neurons and astrocytes. Apparently opposed to that, neuronal action potentials (APs) or spikes represent clearly digital events, like the yes/no or 1/0 of a Turing machine. However, spikes are rarely uniform, but can vary in amplitude and widths, which has significant, differential effects on transmitter release at the presynaptic terminal, where notwithstanding the quantal (vesicular) release itself is digital. Conversely, at the dendritic site of the postsynaptic neuron, there are numerous analog events of computation. Moreover, synaptic transmission of information is not only neuronal, but heavily influenced by astrocytes tightly ensheathing the majority of synapses in brain (tripartite synapse). At least at this point, LTP and LTD modifying synaptic plasticity and believed to induce short and long-term memory processes including consolidation (equivalent to RAM and ROM in electronic devices) have to be discussed. The present knowledge of how the brain stores and retrieves memories includes a variety of options (e.g., neuronal network oscillations, engram cells, astrocytic syncytium). Also epigenetic features play crucial roles in memory formation and its consolidation, which necessarily guides to molecular events like gene transcription and translation. In conclusion, brain computation is not only digital or analog, or a combination of both, but encompasses features in parallel, and of higher orders of complexity.
Keywords: analog-digital computation; artificial and biological intelligence; bifurcations; cellular computation; engrams; learning and memory; molecular computation; network oscillations.
Copyright © 2023 Gebicke-Haerter.

Researchers at CHU Sainte-Justine and Université de Montréal have discovered a new mechanism involved in the expression of Down syndrome, one of the main causes of intellectual disability and congenital heart defects in children. The study’s findings were published today in Current Biology.
Down syndrome (SD), also called trisomy 21 syndrome, is a genetic condition that affects approximately one in every 800 children born in Canada. In these individuals, many genes are expressed abnormally at the same time, making it difficult to determine which genes contribute to which differences.
Professor Jannic Boehm’s research team focused on RCAN1, a gene that is overexpressed in the brains of fetuses with Down syndrome. The team’s work provides insights into how the gene influences the way the condition manifests itself.
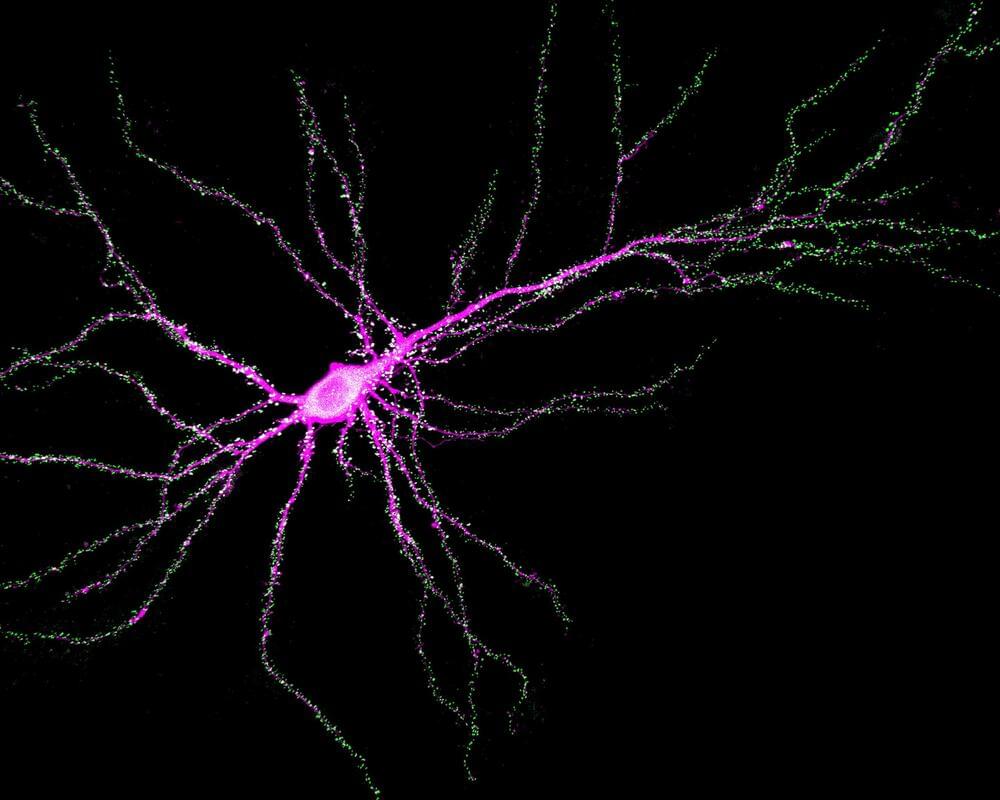
Johns Hopkins Medicine neuroscientists say they have found a new function for the SYNGAP1 gene, a DNA sequence that controls memory and learning in mammals, including mice and humans.
The finding, published in Science, may affect the development of therapies designed for children with SYNGAP1 mutations, who have a range of neurodevelopmental disorders marked by intellectual disability, autistic-like behaviors, and epilepsy.
In general, SYNGAP1, as well as other genes, control learning and memory by making proteins that regulate the strength of synapses—the connections between brain cells.
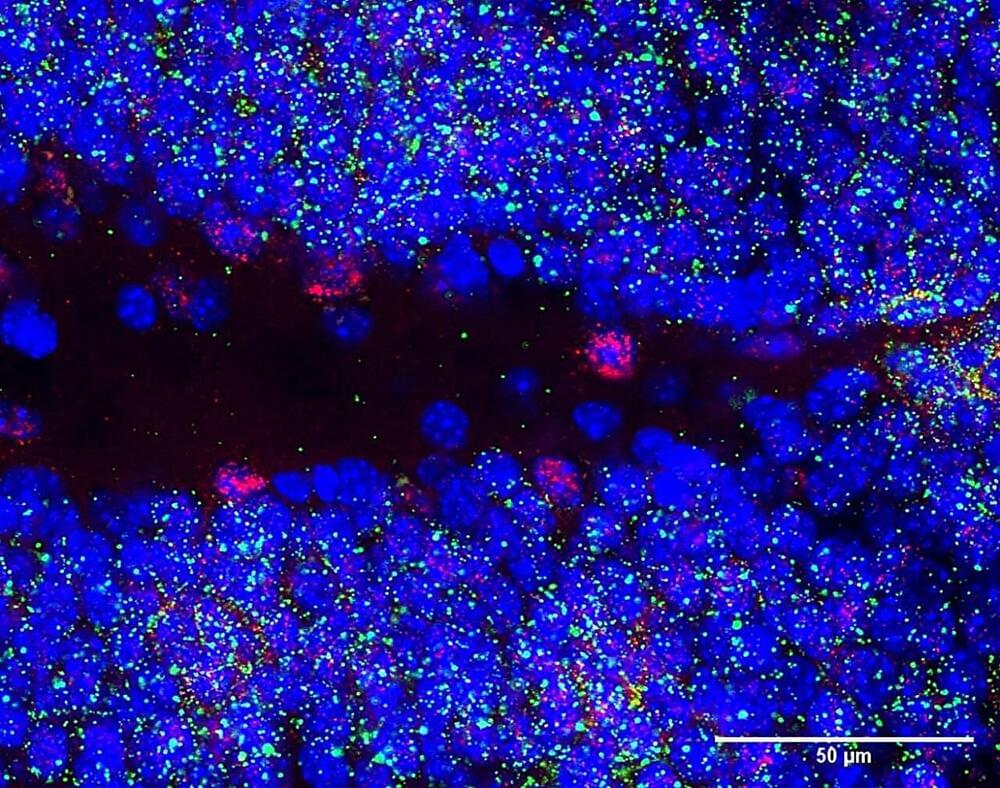
Researchers at the Centre for Genomic Regulation (CRG) have found that the Snhg11 gene is critical for the function and formation of neurons in the hippocampus. Experiments with mice and human tissues revealed that the gene is less active in brains with Down syndrome, potentially contributing to the memory deficits observed in people living with the condition. The findings are published in the journal Molecular Psychiatry.
Traditionally, much of the focus in genomics has been on protein-coding genes, which in humans constitute around just 2% of the entire genome. The rest is “dark matter,” including vast stretches of non-coding DNA sequences that do not produce proteins but are increasingly recognized for their roles in regulating gene activity, influencing genetic stability, and contributing to complex traits and diseases.
Snhg11 is one gene found in the dark matter. It is a long non-coding RNA, a special type of RNA molecule that is transcribed from DNA but does not encode for a protein. Non-coding RNAs are important regulators of normal biological processes, and their abnormal expression has been previously linked to the development of human diseases, such as cancer. The study is the first evidence that a non-coding RNA plays a critical role in the pathogenesis of Down syndrome.

Discount Links: Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…irgwc=1Use Code: CONQUERAGINGNAD+…
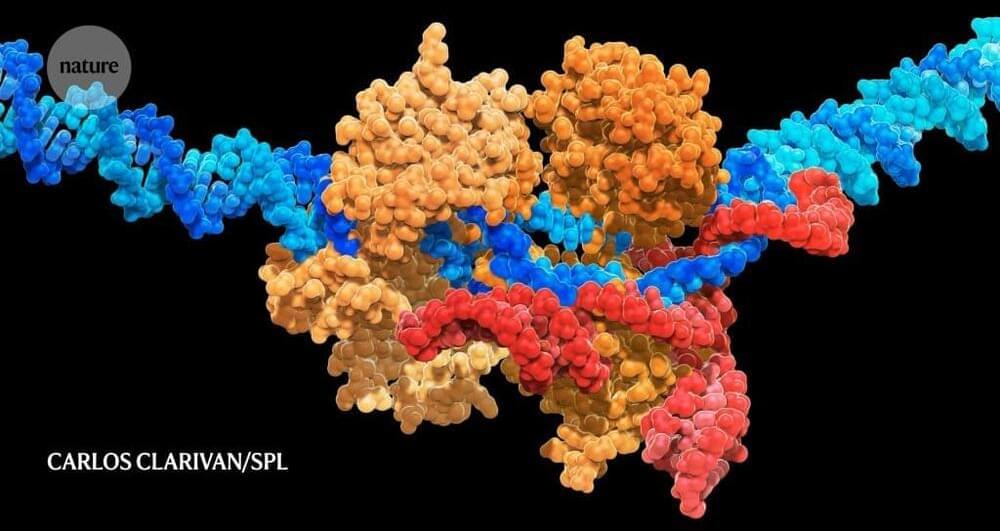
Strategies differ, but there are some gene edits that all researchers agree must underpin any universal stem-cell-derived therapy. There is also widespread consensus that the optimal product should incorporate as few edits as possible, both to minimize the potential for unintended genetic consequences and to streamline manufacturing and regulatory approval.
Beyond that, the scientific community is divided. The complexities of the immune system have fuelled spirited debates over the exact genetic manipulations necessary to create a cell therapy that is both capable of bypassing immune defences and delivering meaningful health benefits.
“The immune system is pervasive and persistent,” says Charles Murry, a cardiovascular pathologist at the University of Washington in Seattle and chief executive of StemCardia in Seattle, one of a growing number of biotechnology companies developing gene-editing strategies to overcome immune barriers in regenerative cell treatments.
Professor Ronjon Nag presents about his project on AI and healthcare, aiming at creating a multi-faceted approved therapy for extending lifespan and curing aging.
Dr. Ronjon Nag is an inventor, teacher and entrepreneur. He is an Adjunct Professor in Genetics at the Stanford School of Medicine, becoming a Stanford Distinguished Careers Institute Fellow in 2016. He teaches AI, Genes, Ethics, Longevity Science and Venture Capital. He is a founder and advisor/board member of multiple start-ups and President of the R42 Group, a venture capital firm which invests in, and creates, AI and Longevity companies. As an AI pioneer of smartphones and app stores, his companies have been sold to Apple, BlackBerry, and Motorola. More recently he has worked on the intersection of AI and Biology. He has numerous interests in the intersection of AI and Healthcare including being CEO of Agemica.ai working on creating a therapy for aging.
Please note that the links below are affiliate links, so we receive a small commission when you purchase a product through the links. Thank you for your support!
=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=*=
~*~ ProHealth, DoNotAge, RenueByScience~*~ Discount Coupon CODE: REVERSE
🌏ProHealth Longevity 15% OFF All Products https://prohealth.pxf.io/REVERSEAGING
Trans-Resveratrol 500mg Caps https://prohealth.pxf.io/TransResvera…
TMG Pro Powder (100 grams) https://prohealth.pxf.io/TMG100
Calcium AKG 500mg Caps https://prohealth.pxf.io/CaAKG
Full Spectrum Apigenin https://prohealth.pxf.io/apigenin.
Green Tea EGCG Extreme https://prohealth.pxf.io/
Berberine Pro 600 mg Caps https://prohealth.pxf.io/Berberine.
🧬 DoNotAge 10% OFF All Products http://tinyurl.com/wk688hav.
Pure Vitamin D3, K2 \& Magnesium http://tinyurl.com/ywxx3j7c.
Pure Spermidine http://tinyurl.com/yc2xbp65 Pure Fisetin http://tinyurl.com/3vhtt7a3
SIRT6 Activator http://tinyurl.com/mss7vrrn.
🔬Renue By Science 10% OFF All Products https://bit.ly/3pAFwDY