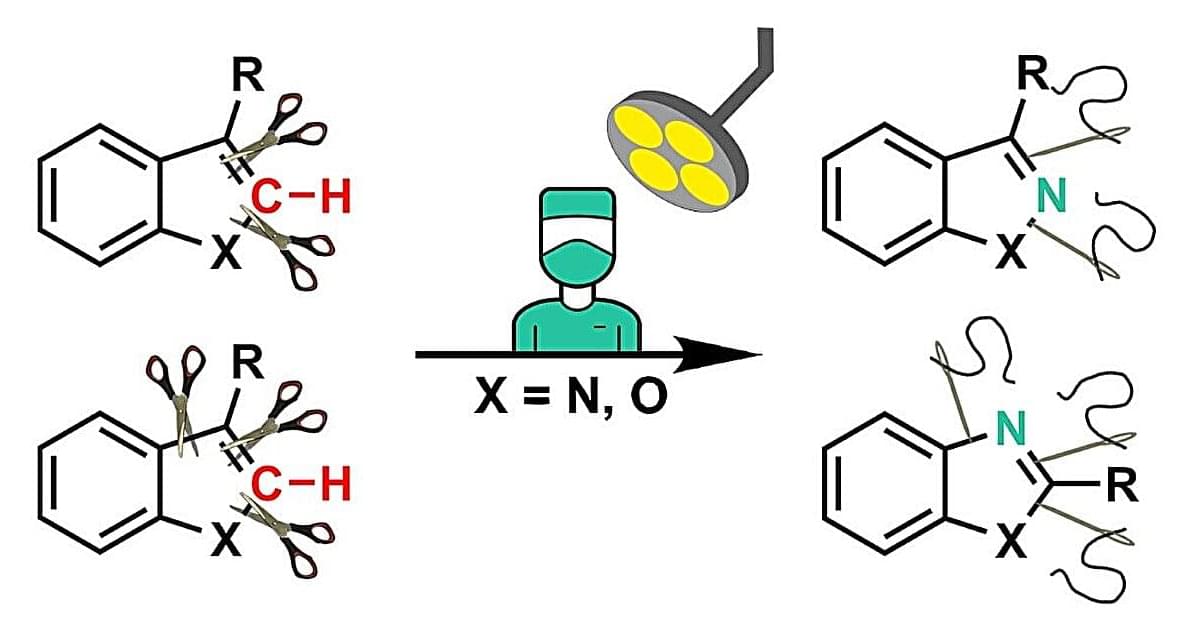
PFOS, also known as “forever chemicals,” are synthetic compounds popular for several commercial applications, like making products resistant to stains, fire, grease, soil and water. They have been used in non-stick cookware, carpets, rugs, upholstered furniture, food packaging and firefighting foams deployed at airports and military airfields.
PFOS (perfluorooctane sulfonate or perfluorooctane sulfonic acid) are part of the larger class of forever chemicals called PFAS (per-and polyfluoroalkyl substances.) Both types have been linked to a variety of health issues, including liver disease, immune system malfunction, developmental issues and cancer.
Because of their widespread use, PFOS are found in soil, agricultural products and drinking water sources, presenting a health risk. Xiaoguang Meng and Christos Christodoulatos, professors at the Department of Civil, Environmental and Ocean Engineering at Stevens Institute of Technology, and Ph.D. student Meng Ji working in their lab, wanted to identify the most efficient way to remove these toxins from the water.