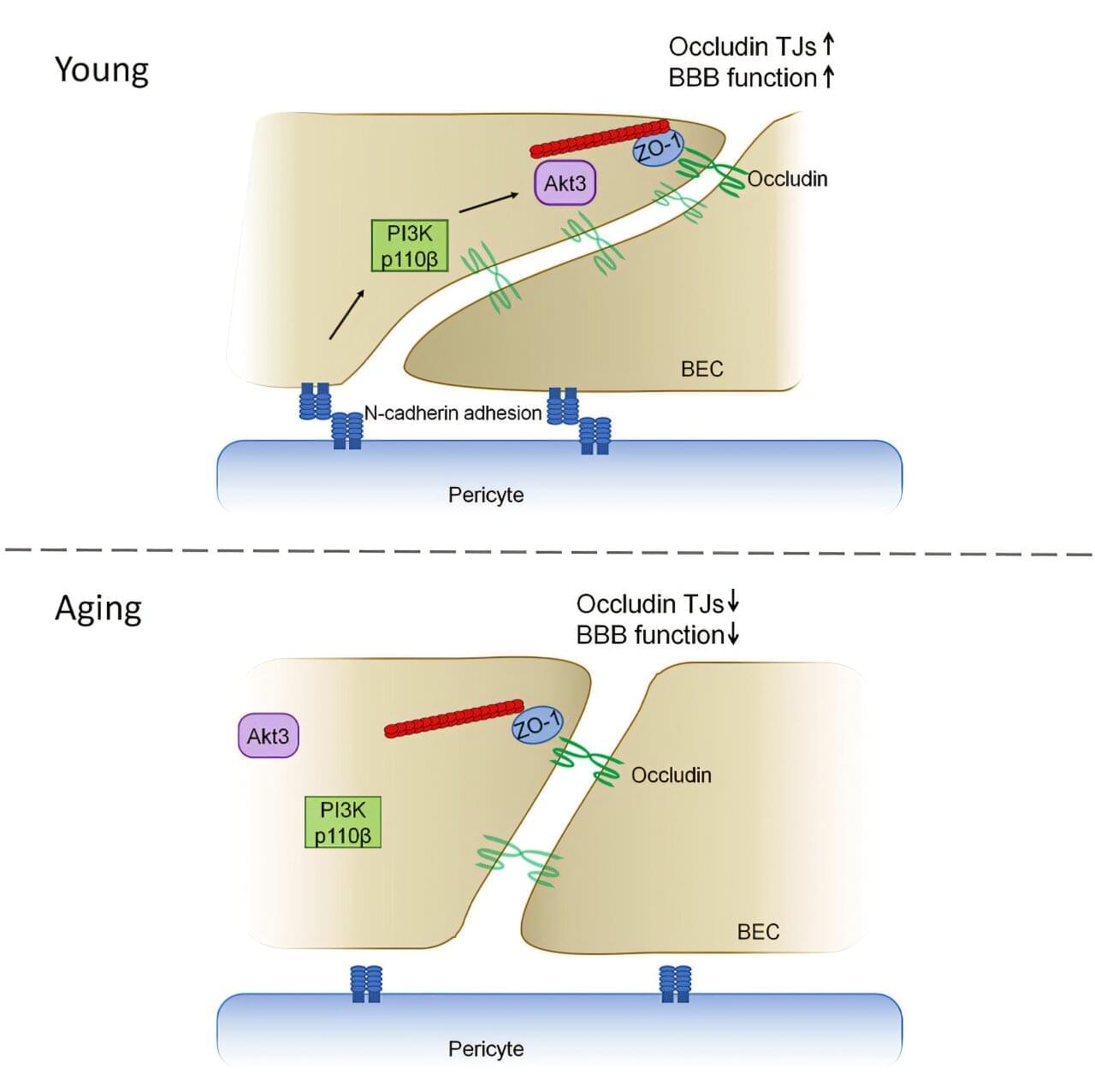
A new study from researchers at the University of Illinois Chicago reveals how the blood-brain barrier gets leakier with age, contributing to memory deficits. The study, published in Cell Reports, uncovered the molecular mechanisms behind this process and could provide new therapeutic targets to address cognitive decline earlier in the aging process.
The blood-brain barrier is a layer of cells lining the brain’s blood vessels that keep viruses, bacteria and toxins out while allowing helpful nutrients and chemicals in. A key structure of the blood-brain barrier are tight junctions that act as bridges between cells, restricting entry of molecules. A protein called occludin helps fulfill this essential role.
“It’s a highly regulatable process that allows some molecules to go through and others to remain in circulation,” said Yulia Komarova, UIC associate professor in the department of pharmacology and regenerative medicine at the College of Medicine and senior author of the study. “Basically, it’s a mechanism that separates the central nervous system from everything else.”