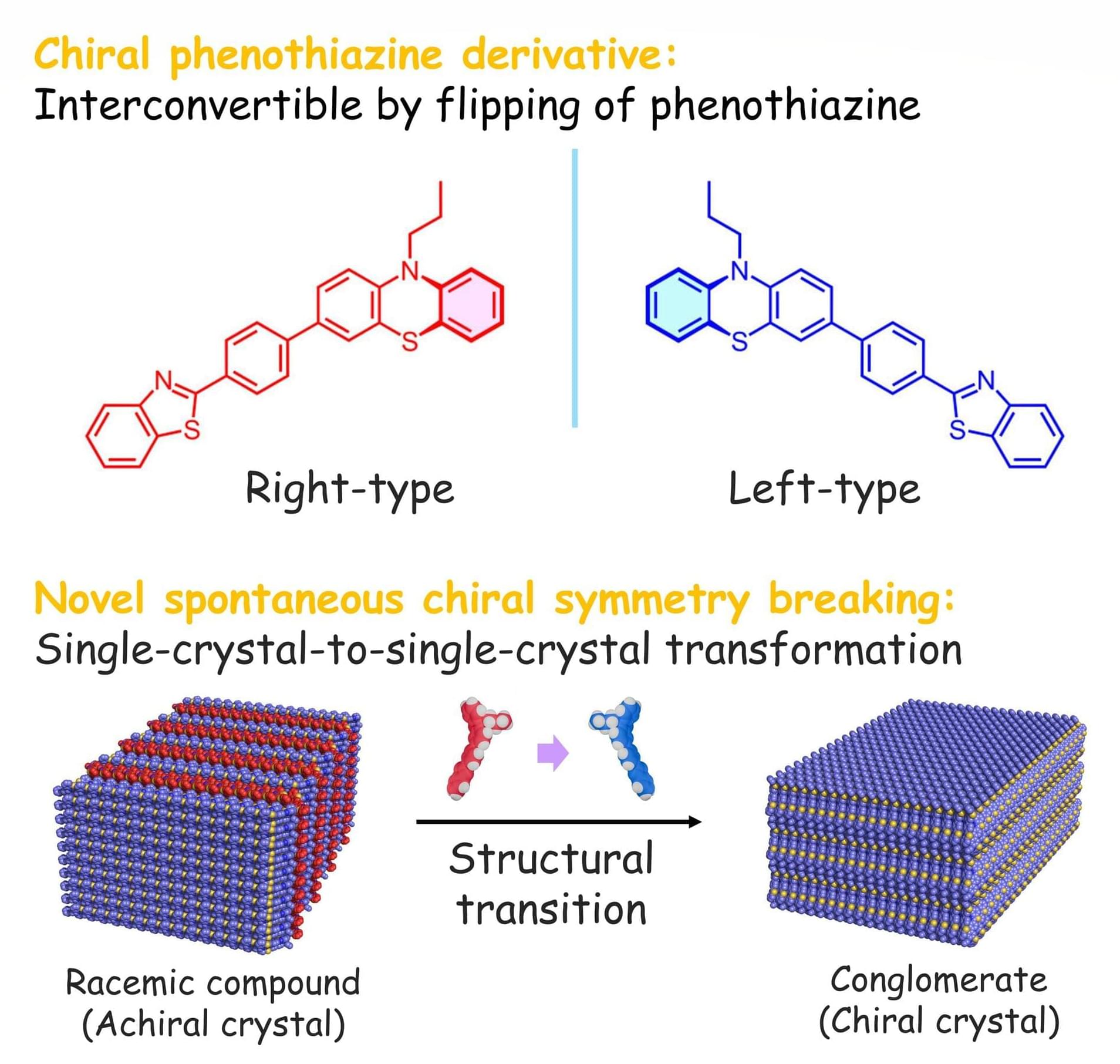
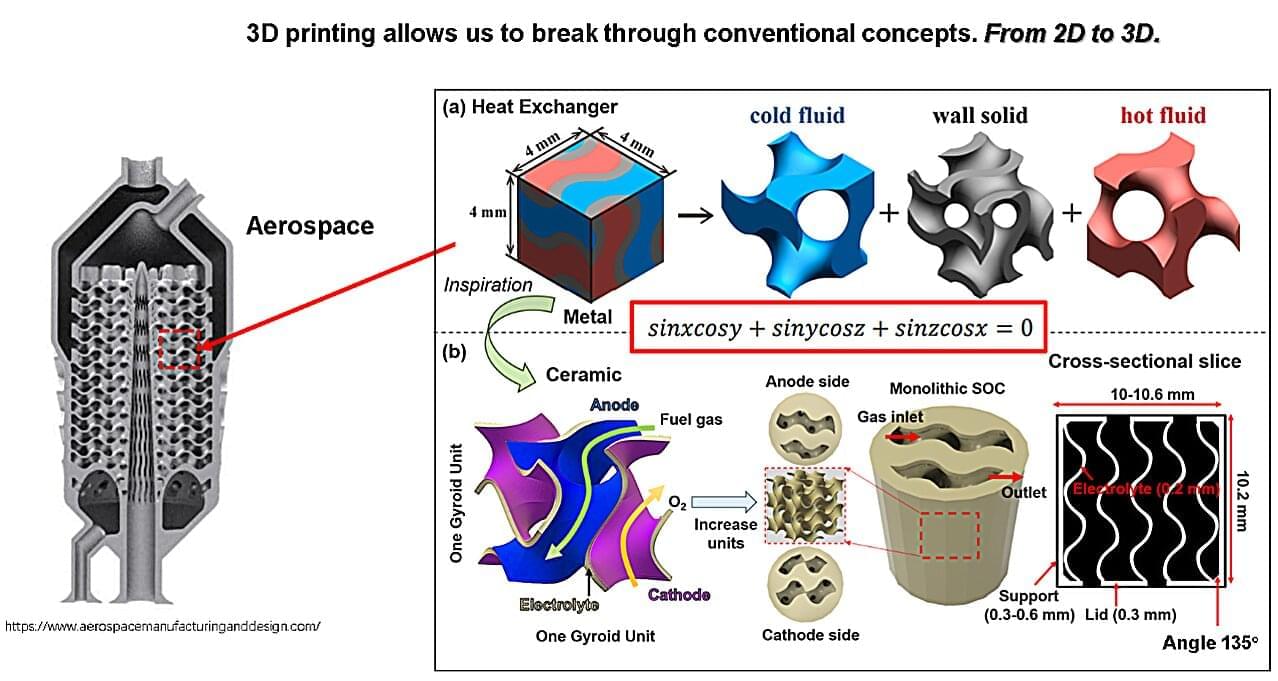
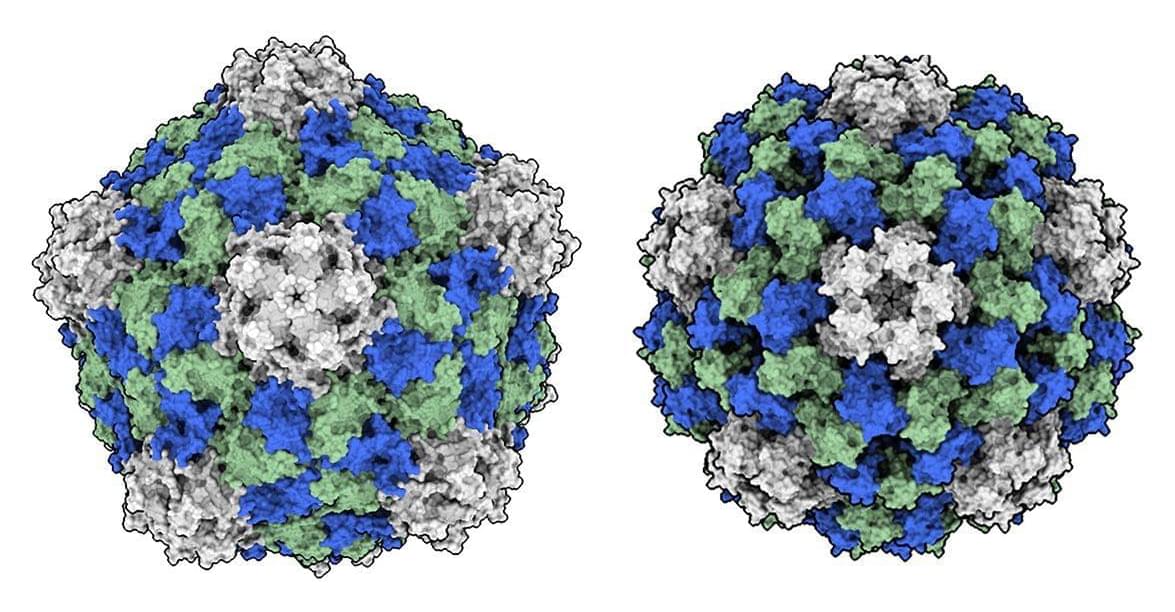
Mitsui & Co. has formally launched a new quantum-enabled chemistry platform, QIDO, in collaboration with U.S.-based Quantinuum and QSimulate. The system, designed to accelerate the discovery of new materials and pharmaceuticals, blends classical and quantum computing resources to streamline complex chemical calculation, according to a story in Nikkei and a Quantinuum blog post.
Quantum computers hold promise for modeling chemical reactions beyond the reach of traditional supercomputers. But fully fault-tolerant systems remain years away, leaving companies searching for ways to extract value from today’s noisy, early-stage machines. QIDO, short for Quantum-Integrated Discovery Orchestrator, attempts to bridge that gap.
The platform runs most computations on powerful classical hardware while sending only the most computationally expensive steps — such as the modeling of strongly correlated electrons — to a quantum computer. This hybrid workflow allows companies to perform higher-precision chemical simulations today, without waiting for fully mature quantum systems, Nikkei reports.