Organic chemistry, the chemistry of carbon compounds, is the basis of all life on Earth. However, metals also play a key role in many biochemical processes. When it comes to “marrying” large, heavy metal atoms with light organic compounds, nature often relies on a specific group of chemical structures: porphyrins. These molecules form an organic ring; in its center, individual metal ions such as iron, cobalt, or magnesium can be “anchored.”
The porphyrin framework forms the basis for hemoglobin in human blood, photosynthetic chlorophyll in plants, and numerous enzymes. Depending on which metal is captured by the porphyrin, the resulting compounds can display a wide range of chemical and physical properties. Chemists and materials scientists have long sought to exploit this flexibility and functionality of porphyrins, including for applications in molecular electronics.
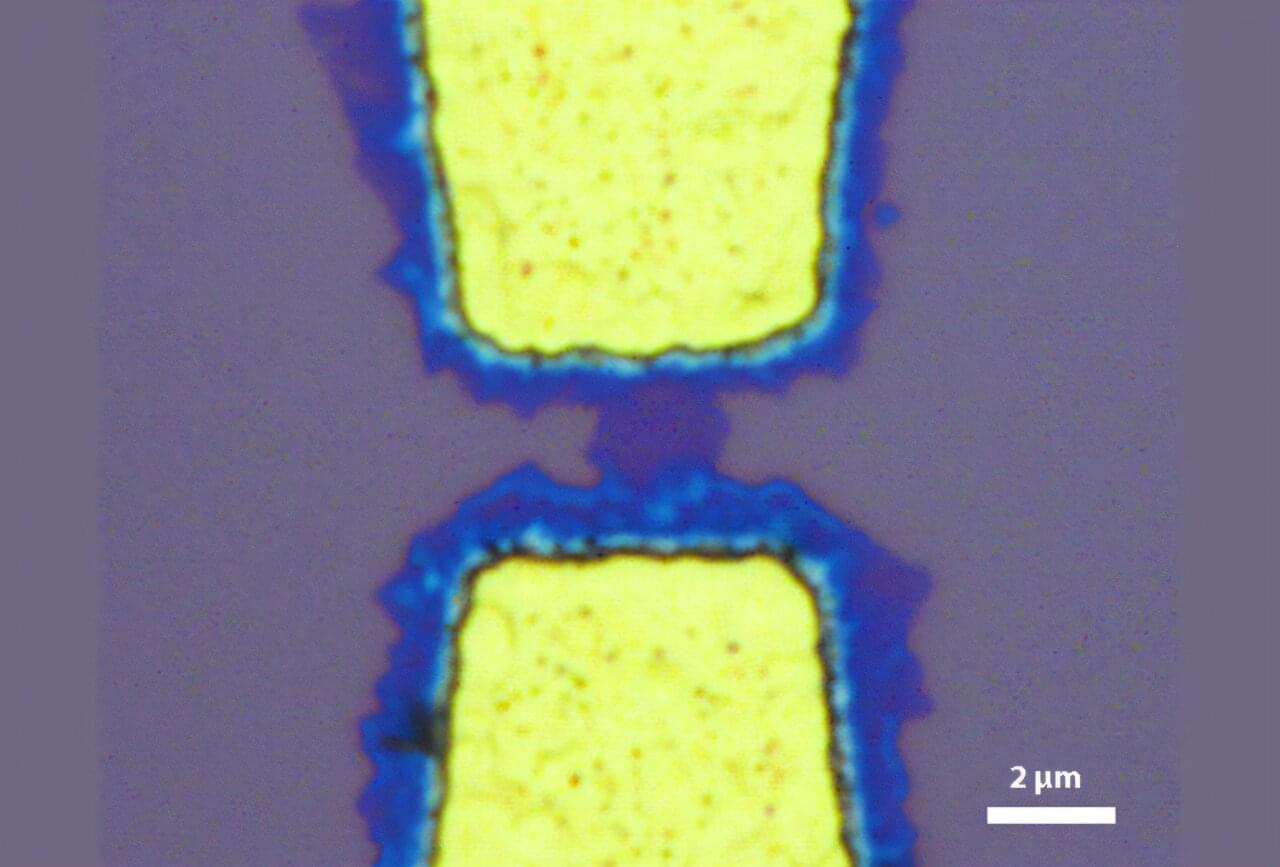
However, for electronic components —even molecular ones—to function, they must be connected to each other. Wiring up individual molecules is no easy task. But this is precisely what researchers at Empa’s nanotech@surfaces laboratory have achieved, in collaboration with synthetic chemists from the Max Planck Institute for Polymer Research.