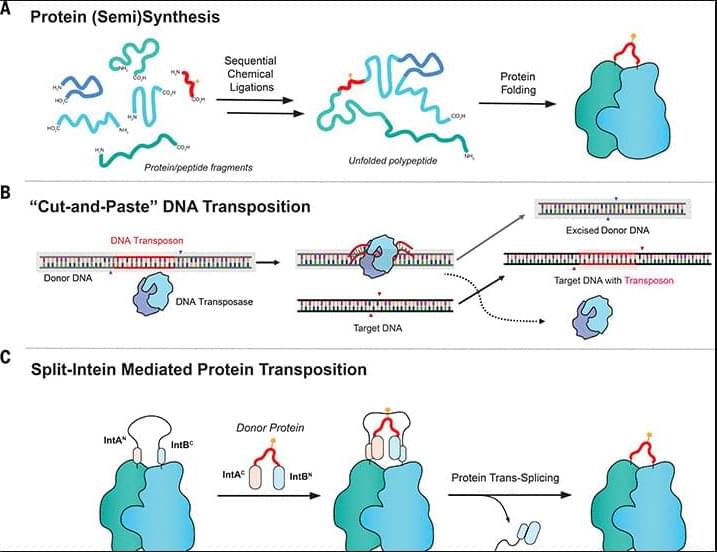
Protein engineering through the ligation of polypeptide fragments has proven enormously powerful for studying biochemical processes. In general, this strategy necessitates a final protein-folding step, constraining the types of systems amenable to the approach. Here, we report a method that allows internal regions of target proteins to be replaced in a single operation. Conceptually, our system is analogous to a DNA transposition reaction but uses orthogonal pairs of engineered split inteins to mediate the editing process. This “protein transposition” reaction is applied to several systems, including folded protein complexes, allowing the efficient introduction of a variety of noncoded elements. By carrying out a molecular “cut and paste” under native protein-folding conditions, our approach substantially expands the scope of protein semisynthesis.