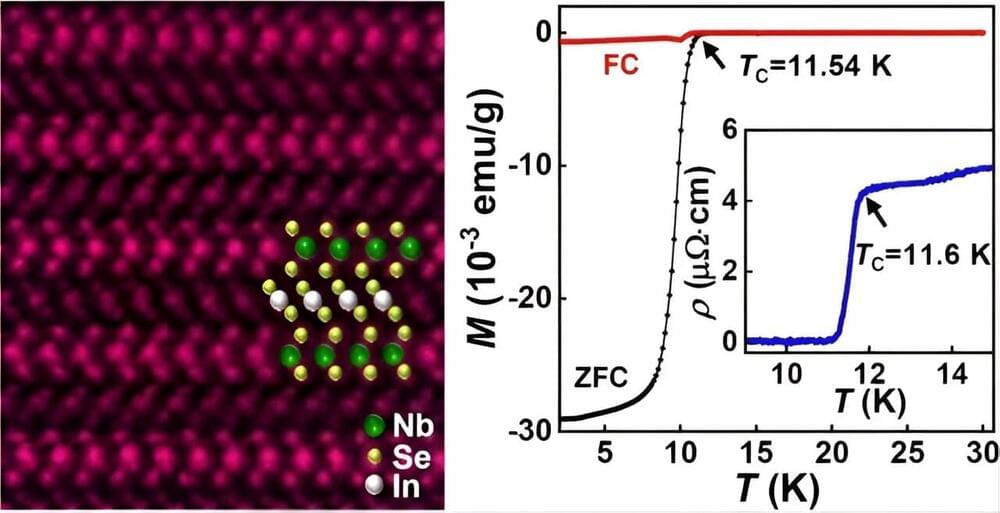
In the XXI century, the world of orbital launchers has started a revolution, a fundamental change of paradigm: the replacement of expendable rockets with reusable ones is well underway. This presentation summarizes the situation at the beginning of year 2024.
A short bio.
Alberto Cavallo is an Electrical Engineer, graduated at the Politecnico di Torino in 1985. He began his activity with designing electric systems in Fiat Engineering, the engineering and construction company of the FIAT Group, moving soon to control and automation systems in the same company. He was involved in all business areas of the company, which included revamping and new projects of car factories for the FIAT Group as well as large infrastructures, power and cogeneration plants for external clients. Among the projects of that time were the new FIAT factories in Melfi and Pratola Serra, the high speed railways Torino-Milano and Bologna-Firenze, the district heating system of Torino Sud, combined cycle power plants for several hundred megawatts in Italy and in Brazil. Since Fiat Engineering was transferred from the FIAT Group to a new EPC group and then merged with a large EPC company in Milan, he has been involved in large oil and gas and petrochemical projects all over the world. Besides his professional activity, he has always taken part in several cultural activities. He was a member of the Associations of Alumni of the Liceo Classico Vittorio Alfieri of Turin, active in promoting humanistic culture as well as its connection to the technical and scientific area. He manages his own website www.eurinome.it (in Italian only) about philosophy, science and politics/geopolitics. Due to this he got in contact with Adriano Autino and his TDF, then becoming one of the founding members of Space Renaissance International. Besides several papers in his professional area he has written several articles for his own site, for TDF and SRI, coauthoring the book “Three Theses for the Space Renaissance” with Adriano Autino and Patrick Q. Collins. He is currently member of the Board of SRI.