Researchers from Northwestern and Georgia Tech developed an organic electrochemical neuron that fires at human-like frequencies, enabling real-time tactile signal processing. Their system integrates artificial neurons, touch receptors and synapses.
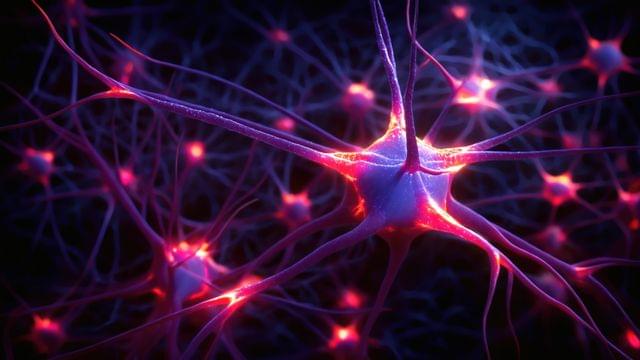

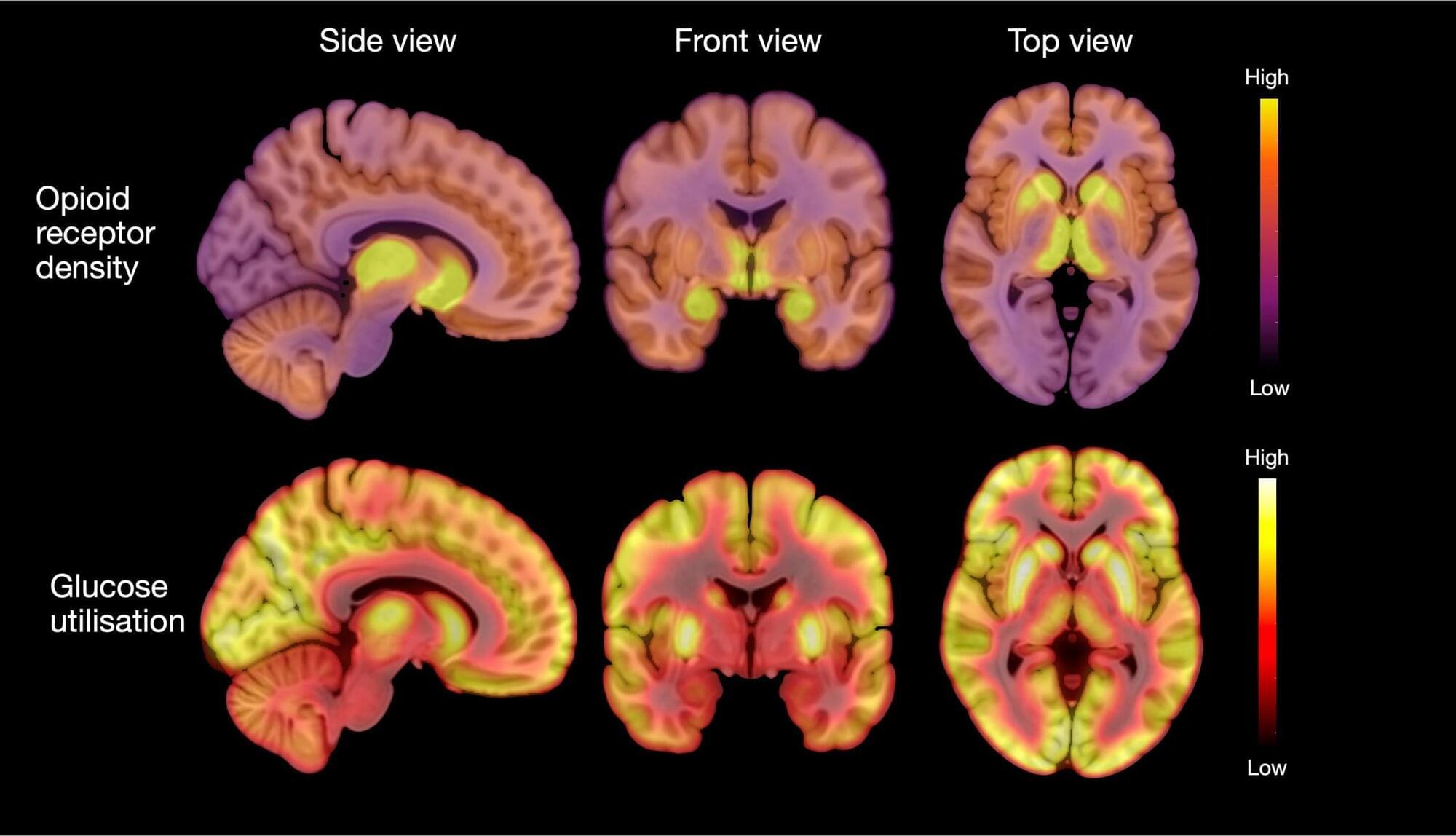
New research found that individuals with anorexia nervosa have elevated opioid neurotransmitter.
A neurotransmitter is a chemical substance that transmits signals across a synapse from one neuron to another in the nervous system. These chemicals play a crucial role in the functioning of the brain and body, influencing everything from mood, sleep, and learning to heart rate, anxiety, and fear. Common neurotransmitters include dopamine, serotonin, acetylcholine, and norepinephrine. They bind to specific receptors on the surface of neurons, triggering various physiological responses and allowing for the communication that underpins all neural activities. Imbalances in neurotransmitter levels can lead to neurological disorders or mental health issues, making them a central focus of study in both medicine and psychology.

However, a new study proves that hydrogen bonds can effectively link spin centers, enabling easier assembly of molecular spin qubits. This discovery could transform quantum material development by leveraging supramolecular chemistry.
A Light-Driven Approach to Spin Qubits
Qubits are the fundamental units of information in quantum technology. A key challenge in developing practical quantum applications is determining what materials these qubits should be made of. Molecular spin qubits are particularly promising for molecular spintronics, especially in quantum sensing. In these systems, light can stimulate certain materials, creating a second spin center and triggering a light-induced quartet state.

A study by cognitive neuroscientists at SISSA investigated how the human brain processes space and time, uncovering that these two types of information are only partially connected.
Imagine a swarm of fireflies flickering in the night. How does the human brain process and integrate information about both their duration and spatial position to form a coherent visual experience? This question was the focus of research by Valeria Centanino, Gianfranco Fortunato, and Domenica Bueti from SISSA’s Cognitive Neuroscience group, published in Nature Communications
<em> Nature Communications </em> is an open-access, peer-reviewed journal that publishes high-quality research from all areas of the natural sciences, including physics, chemistry, Earth sciences, and biology. The journal is part of the Nature Publishing Group and was launched in 2010. “Nature Communications” aims to facilitate the rapid dissemination of important research findings and to foster multidisciplinary collaboration and communication among scientists.
The data inscribed into the crystal is carefully annotated with universal elements like hydrogen, oxygen, carbon, and nitrogen, as well as the four DNA bases—adenine, cytosine, guanine, and thymine—that make up the genetic code. Additionally, the molecular structure of DNA and the arrangement of genes within chromosomes are depicted, offering clear instructions on how to interpret the genetic information stored within.
However, it is important to note that the 5D memory crystals require a highly specialized skill set and advanced equipment to inscribe and read the data stored within the crystals, so those looking to re-establish the human race after an extinction event may have to refer to more traditional means.
The crystal, made from fused quartz, is one of the most chemically and thermally resilient materials known on Earth, and can endure temperatures as high as 1000°C, resist direct impact forces up to 10 tons per square centimeter, and is unaffected by long-term exposure to cosmic radiation. The longevity and storage capacity of the 5D memory crystal earned it a Guinness World Record in 2014 for being the most durable data storage material ever created.
According to management consulting firm BCG, only around half of all aluminum beverage cans are recycled in the United States, which is far behind countries such as Germany. What’s more, aluminum has one of the highest recycling rates in the U.S. — only around 19% of the durable goods sold in the U.S. are recycled, including only 14% of plastic containers and packaging. The rest is sent to landfills, where it leaches toxic chemicals into the surrounding soil and waterways.
New processes such as the one developed by the MIT researchers can hopefully make a difference in those numbers.
“We’re not just preventing waste,” said John H. Lienhard, another one of the researchers. “This membrane technology also enables a circular economy for aluminum, which could reduce the need for new mining and help mitigate some of the industry’s environmental footprint.”
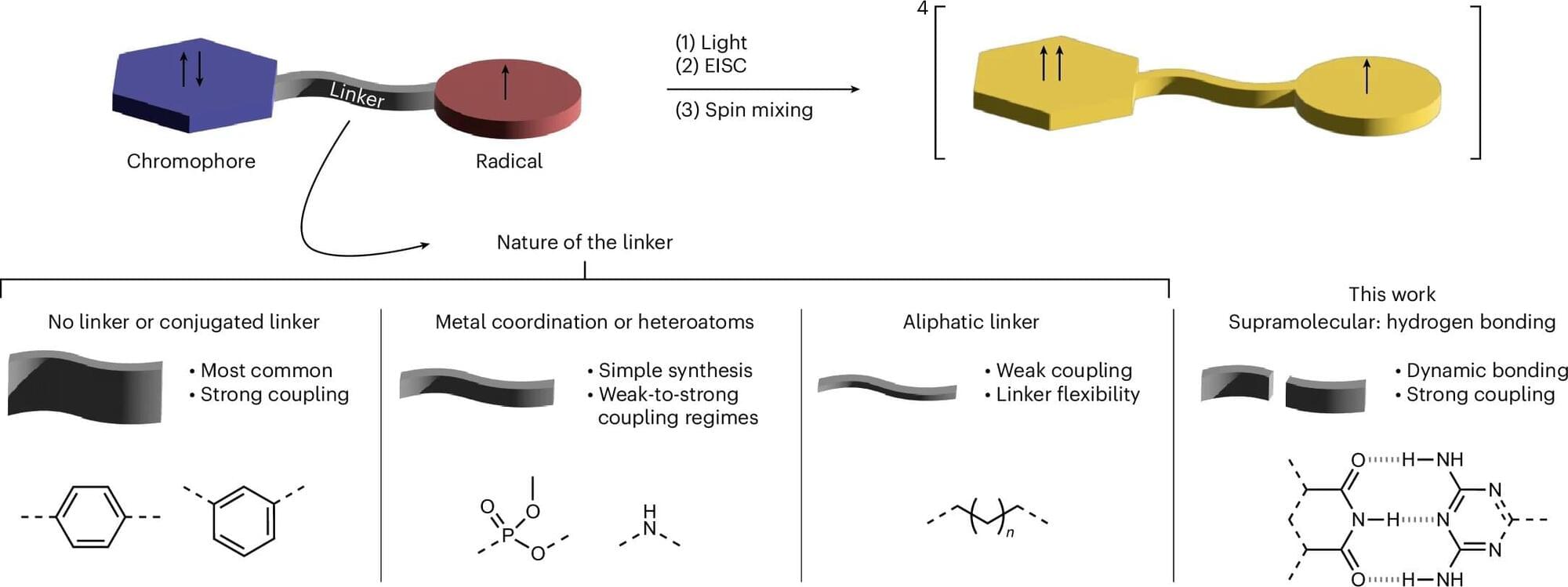
A Franco-German research team, including members from the University of Freiburg, shows that supramolecular chemistry enables efficient spin communication through hydrogen bonds. The work is published in the journal Nature Chemistry.
Qubits are the basic building blocks of information processing in quantum technology. An important research question is what material they will actually consist of in technical applications. Molecular spin qubits are considered promising qubit candidates for molecular spintronics, in particular for quantum sensing. The materials studied here can be stimulated by light; this creates a second spin center and, subsequently, a light-induced quartet state.
Until now, research has assumed that the interaction between two spin centers can only be strong enough for successful quartet formation if the centers are covalently linked. Due to the high effort required to synthesize covalently bonded networks of such systems, their use in application-related developments in the field of quantum technology is severely limited.
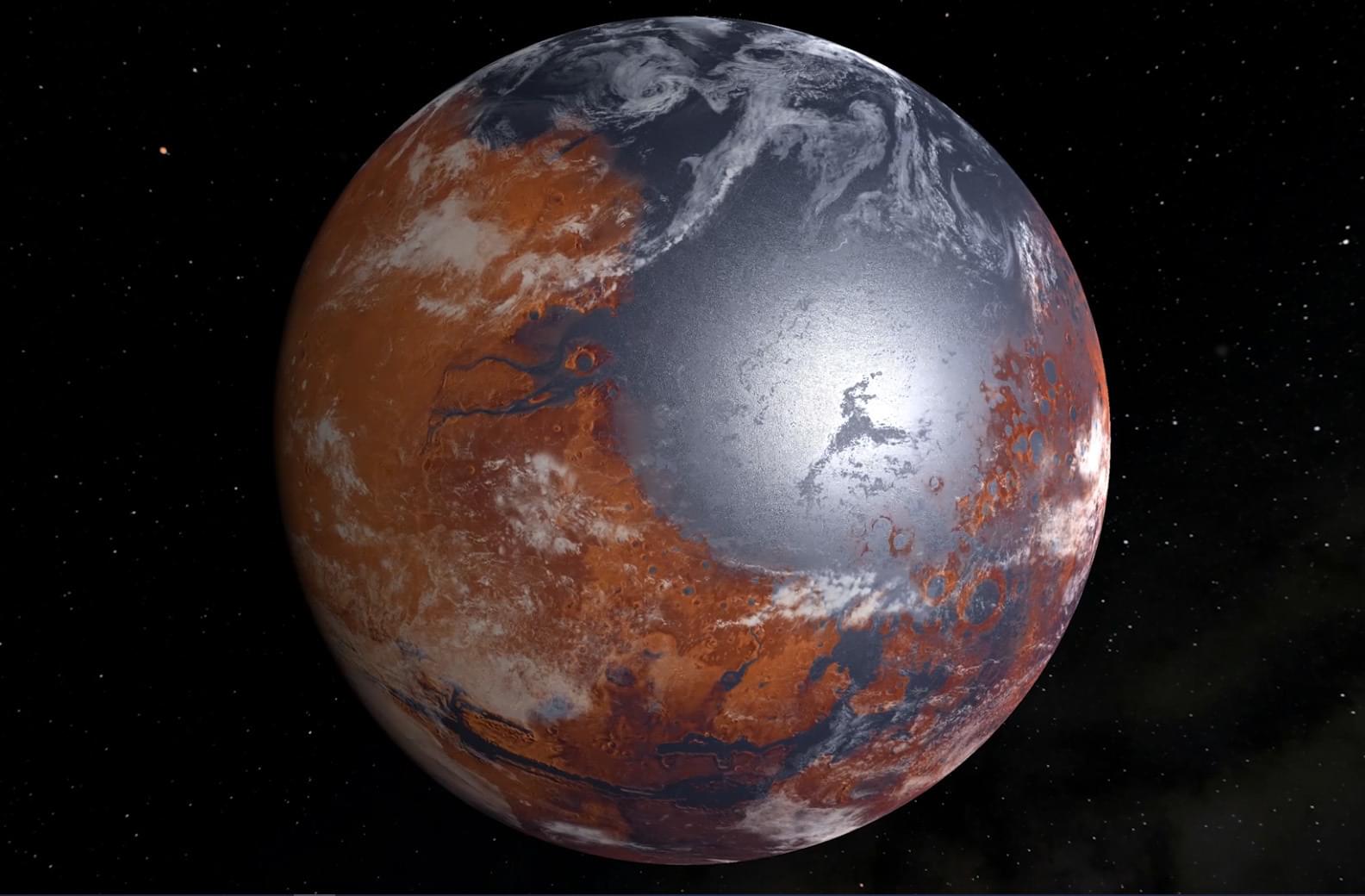
Did Mars have lakes and rivers during a single period or over separate periods? This is what a recent study published in Nature Geoscience hopes to address as an international team of researchers investigated whether Mars experienced a single event of liquid water on its surface, or many events spread over millions of years. This study has the potential to help scientists better understand the early conditions on Mars and whether these conditions were suitable to support life as we know it.
“Early Mars is a lost world, but it can be reconstructed in great detail if we ask the right questions,” said Dr. Robin Wordsworth, who is a Gordon McKay Professor of Environmental Science and Engineering at Harvard University and a co-author on the study. “This study synthesizes atmospheric chemistry and climate for the first time, to make some striking new predictions – which are testable once we bring Mars rocks back to Earth.”
For the study, the researchers used a series of computer models to simulate how the atmosphere on Mars billions of years ago potentially reacted to surface water-rock interactions and climate changes over time. The goal was to ascertain whether Mars experienced a single event of liquid water on its surface, or a series of events spread over millions of years with periods of dryness in between them.

Dr. Armour, in 1991, discovered that the heart has its “little brain” or “intrinsic cardiac nervous system.” This “heart brain” is composed of approximately 40,000 neurons that are alike neurons in the brain, meaning that the heart has its own nervous system. In addition, the heart communicates with the brain in many methods: neurologically, biochemically, biophysically, and energetically. The vagus nerve, which is 80% afferent, carries information from the heart and other internal organs to the brain. Signals from the “heart brain” redirect to the medulla, hypothalamus, thalamus, and amygdala and the cerebral cortex. Thus, the heart sends more signals to the brain than vice versa. Research has demonstrated that pain perception is modulated by neural pathways and methods targeting the heart such as vagus nerve stimulation and heart-rhythm coherence feedback techniques. The heart is not just a pump. It has its neural network or “little brain.” The methods targeting the heart modulate pain regions in the brain. These methods seem to modulate the key changes that occur in the brain regions and are involved in the cognitive and emotional factors of pain. Thus, the heart is probably a key moderator of pain.

Researchers at Cornell University on Monday showcased a pair of bio-inspired robotics running on a hydraulic fluid-powered battery. The redox flow battery (RFB) also mimics biological functions, as it releases electrolytic fluids, which dissolve to create energy through chemical reaction.
The first two robots on display are a modular worm and a jellyfish, designed by the Cornell Engineering labs. The batteries powering these systems utilize embodied energy, “an approach that incorporates power sources into the body of a machine, to reduce its weight and cost,” according to the school.
Mechanical and aerospace engineering Professor Rob Shepherd describes the underlying technology thusly: “There are a lot of robots that are powered hydraulically, and we’re the first to use hydraulic fluid as the battery, which reduces the overall weight of the robot, because the battery serves two purposes, providing the energy for the system and providing the force to get it to move.”