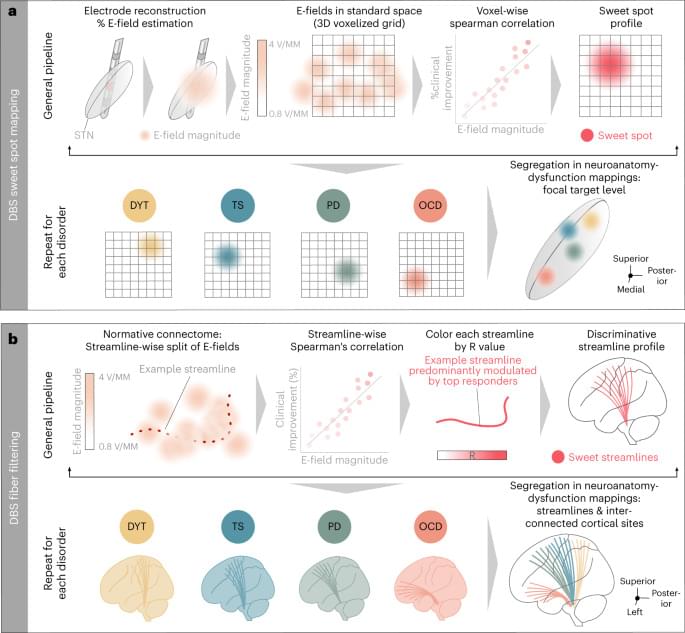
Hollunder et al. identify networks where deep brain stimulation reduces symptoms for Parkinson’s disease, Tourette’s syndrome, dystonia and obsessive-compulsive disorder. This revealed a fronto-rostral topography that segregates the frontal cortex.

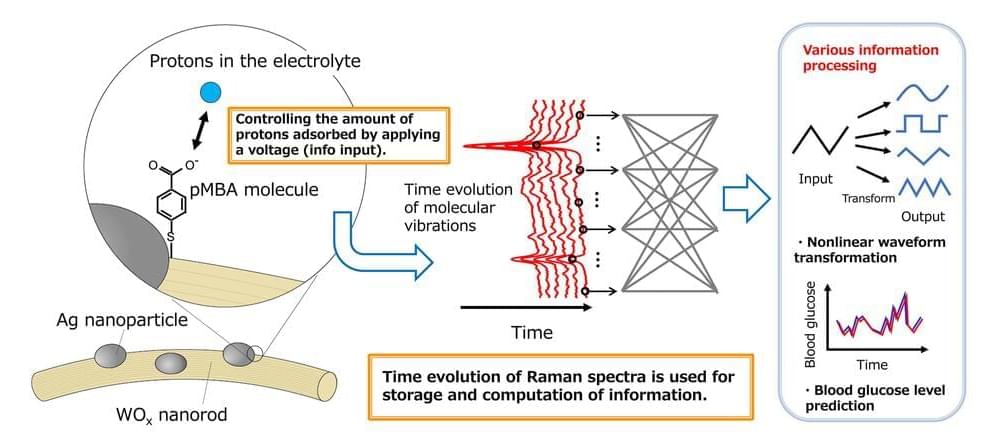
A collaborative research team from NIMS and Tokyo University of Science has successfully developed an artificial intelligence (AI) device that executes brain-like information processing through few-molecule reservoir computing. This innovation utilizes the molecular vibrations of a select number of organic molecules.
By applying this device for the blood glucose level prediction in patients with diabetes, it has significantly outperformed existing AI devices in terms of prediction accuracy.
The work is published in the journal Science Advances.

According to a big German study, those in middle or older age today have an elevated idea of “old” compared to previous generations.” This mirrors increases in life expectancy, especially for the better-off half of the population in rich countries.
Research is finding ways to extend animal lifespans but regulators are still wary of treating ageing as a disease.
Medically, AI is helping us with everything from identifying abnormal heart rhythms before they happen to spotting skin cancer. But do we really need it to get involved with our genome? Protein-design company Profluent believes we do.
Founded in 2022 in Berkeley, California, Profluent has been exploring ways to use AI to study and generate new proteins that aren’t found in nature. This week, the team trumpeted a major success with the release of an AI-derived protein termed OpenCRISPR-1.
The protein is meant to work in the CRISPR gene-editing system, a process in which a protein cuts open a piece of DNA and repairs or replaces a gene. CRISPR has been actively in use for about 15 years, with its creators bagging the Nobel prize in chemistry in 2020. It has shown promise as a biomedical tool that can do everything from restoring vision to combating rare diseases; as an agricultural tool that can improve the vitamin D content of tomatoes, and slash the flowering time of trees from decades to months; and much more.
In order to terraform new planets, we will need to be able transport entire ecologies & ecosystems through interstellar space in the future. Today we will examine how we could build and maintain such environments inside vast arks, generations ships able to colonize our galaxy, and the challenges these starships will face maintaining not just stores of DNA and genetic material but living organisms which depend heavily on other members of their species and other species to survive and thrive, not least of which is human ourselves. Visit our sponsor, Brilliant: https://brilliant.org/IsaacArthur/ Join this channel to get access to perks: / @isaacarthursfia Visit our Website: http://www.isaacarthur.net Join Nebula: https://go.nebula.tv/isaacarthur Support us on Patreon:
/ isaacarthur Support us on Subscribestar: https://www.subscribestar.com/isaac-a… Group:
/ 1,583,992,725,237,264 Reddit:
/ isaacarthur Twitter:
/ isaac_a_arthur on Twitter and RT our future content. SFIA Discord Server:
/ discord Listen or Download the audio of this episode from Soundcloud: Episode’s Audio-only version:
/ exporting-earth Episode’s Narration-only version:
/ exporting-earth-ships-narration-only Credits: Exporting Earth Episode 150, Season 4 E36 Writers: Isaac Arthur Editors: Darius Said Gregory Leal https://www.gregschool.org/ Jerry Guern Konstantin Sokerin Laura Graham Mark Warburton Matthew Acker Sigmund Kopperud Stuart Graham https://beyondnerva.wordpress.com Producer: Isaac Arthur Cover Artist: Jakub Grygier https://www.artstation.com/jakub_grygier Graphics Team: Darth Biomech https://www.artstation.com/darth_biomech Fishy Tree https://www.deviantart.com/fishytree/ Jarred Eagley Jeremy Jozwik https://www.artstation.com/zeuxis_of_… Katie Byrne Ken York
/ ydvisual Krisitijan Tavcar https://www.miragedereve.com LegionTech Studios Sam McNamara Sergio Boterio https://www.artstation.com/sboterod?f… Narrator: Isaac Arthur Music Manager: Luca DeRosa — [email protected] Music: Dracovallis, “Golden Meadows” https://dracovallis.bandcamp.com/ NJ Mandaville, “Intrumental Background 1”
/ nj-mandaville Kevin Macleod, “Infinite Wonder”
/ @incompetech_kmac Chris Zabriskie, “Candlepower” http://chriszabriskie.com Kai Engel, “Endless Story About Sun and Moon” https://www.kai-engel.com/ Lombus, “Amino” https://lombus.bandcamp.com Aerium, “Windmill Forests”
/ @officialaerium Epic Mountain, “Rising Sky”
/ epicmountain.

Charles Darwin and his followers postulated that random accidental mutations of small effect plus natural selection over long periods would provide sufficient hereditary variation to explain biological diversity. Research since the middle of the twentieth century has unexpectedly shown that living organisms possess many different means of altering their genomes biologically, and these processes have been validated by DNA sequence analysis. In addition, the biological process of interspecific hybridization has become recognized as a major source of rapid speciation and genome amplification. Thus, it is time to shift our basic concept of evolutionary variation from the traditional model of slow change from non-biological sources to a fully biological model of rapid genome reorganization stimulated by challenges to reproduction.
Introduction
In Western society prior to the Enlightenment, there was little disagreement about the origins of biological diversity: it resulted from divine creation of an unchanging panorama of plant and animal species, as explained in Genesis. No thought was given to the idea that living organisms could change their fundamental natures. Even a scientist dedicated to analyzing the nature and classification of life forms, Carl Linnaeus (1707−1778), and one who documented the extinction of fossil organisms, Georges Cuvier (1769−1832), both believed in the fixity of species.
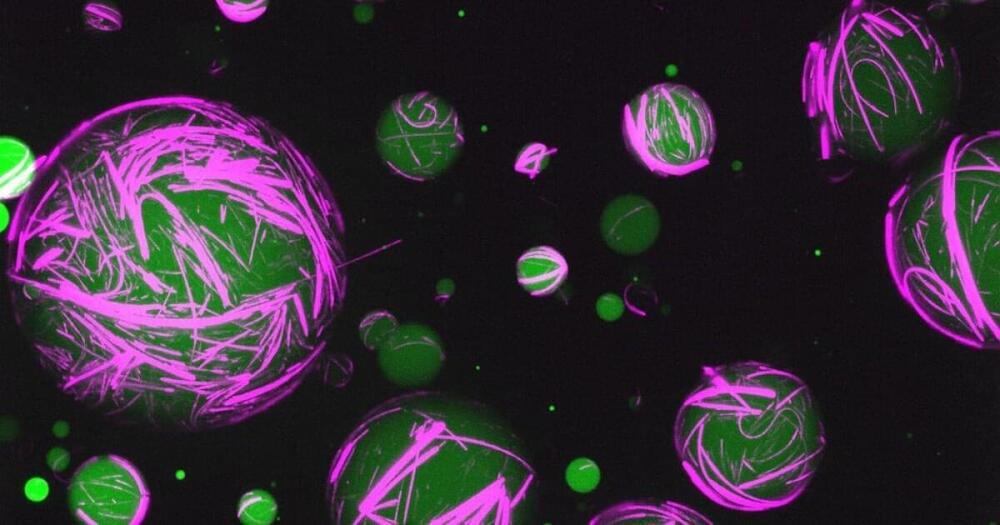
Using DNA and proteins, scientists have created new synthetic cells that act like living cells. Blurring the line between artificial and living materials, these cells can be reprogrammed to perform multiple functions, opening the door to new synthetic biology tech that goes beyond nature’s abilities.
Cells get their structure and stability from their cytoskeleton, a crosslinked framework of proteins that encases and protects other components. Depending on the type of cell, this cytoskeleton can be flexible to different degrees and respond in different ways to their environment, giving cells their specialized abilities.
For the new study, scientists from the University of North Carolina at Chapel Hill developed synthetic, self-assembling cytoskeletons, built out of DNA, peptides and other genetic material.

Advances in generative AI have taken the tech world by storm. Biotech investors are making a big bet that similar computational methods could revolutionize drug discovery.
On Tuesday, ARCH Venture Partners and Foresite Labs, an affiliate of Foresite Capital, announced that they incubated Xaira Therapeutics and funded the AI biotech with $1 billion. Other investors in the new company, which has been operating in stealth mode for about six months, include F-Prime, NEA, Sequoia Capital, Lux Capital, Lightspeed Venture Partners, Menlo Ventures, Two Sigma Ventures and SV Angel.
Xaira’s CEO Marc Tessier-Lavigne, a former Stanford president and chief scientific officer at Genentech, says the company is ready to start developing drugs that were impossible to make without recent breakthroughs in AI. “We’ve done such a large capital raise because we believe the technology is at an inflection point where it can have a transformative effect on the field,” he said.
The single-celled organism Naegleria fowleri ranks among the deadliest human parasites. Matthias Horn and Patrick Arthofer of the University of Vienna’s Center for Microbiology and Environmental Systems Science, along with other researchers, have identified viruses that target this dangerous organism.
Named Naegleriavirus, these belong to the giant viruses, a group known for their unusually large particles and complex genomes. The team details their findings in the prestigious journal, Nature Communications.
Naegleri species are single-celled amoebae, found globally in water bodies. Notably, one species, Naegleria fowleri, thrives in warm waters above 30°C and causes primary amoebic meningoencephalitis (PAM), a rare but almost invariably fatal brain infection. A research team led by Patrick Arthofer and Matthias Horn from the University of Vienna’s Center for Microbiology and Environmental Systems Science (CeMESS) has now isolated giant viruses that infect various Naegleria species.