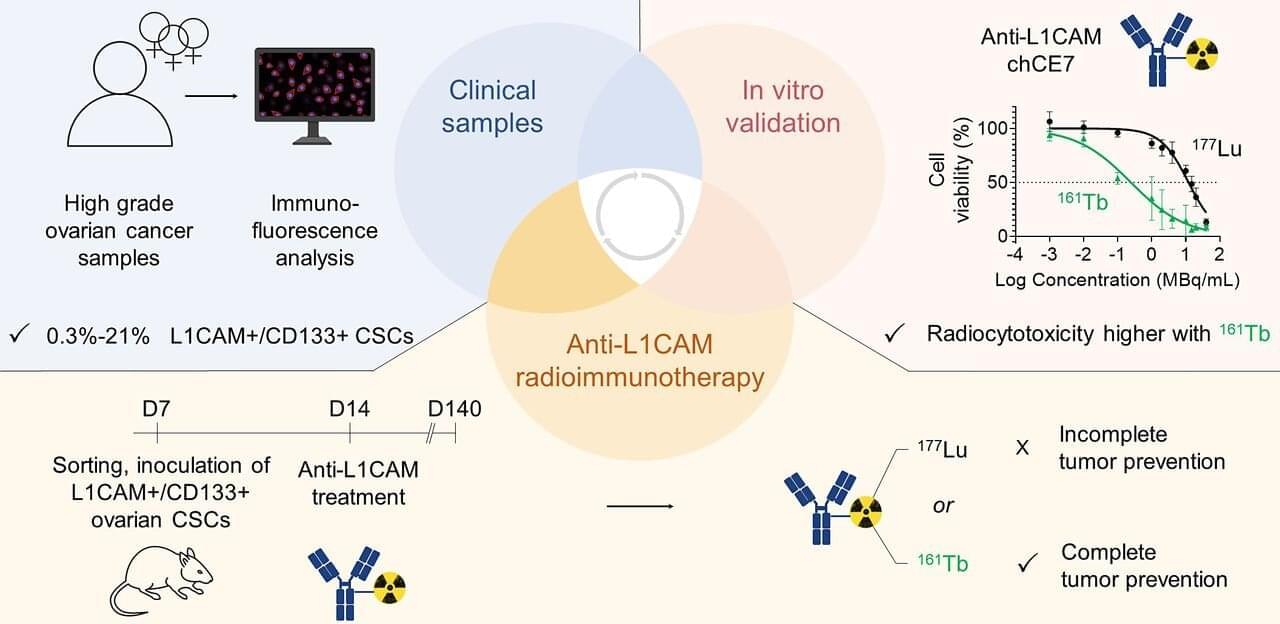
A new radioimmunotherapy approach has been shown to successfully eliminate cancer stem cells (CSCs) in preclinical models of ovarian cancer, outperforming the current gold standard. This research, published in the July issue of The Journal of Nuclear Medicine, lays the foundation for further development of radionuclide therapies targeting CSCs, offering renewed hope for more effective treatment options and improved outcomes for patients.
CSCs are highly tumorigenic, self-renewable cells that play a key role in tumor relapse, metastasis, and therapy resistance. Although the clinical significance of eliminating CSCs is clearly recognized and CSC immunotherapies have been examined in preclinical and clinical evaluations, the development of such therapies remains a challenge.
“Radioimmunotherapy enables precise, target-specific delivery of particulate radiation to cancer-associated antigens, while minimizing off-target accumulation and increasing tumor retention and irradiation, which makes it a promising choice for targeting CSCs,” stated Jürgen Grünberg, Ph.D., senior scientist at the Center for Radiopharmaceutical Sciences, Center for Life Sciences at the Paul Scherrer Institute in Villigen, Switzerland.