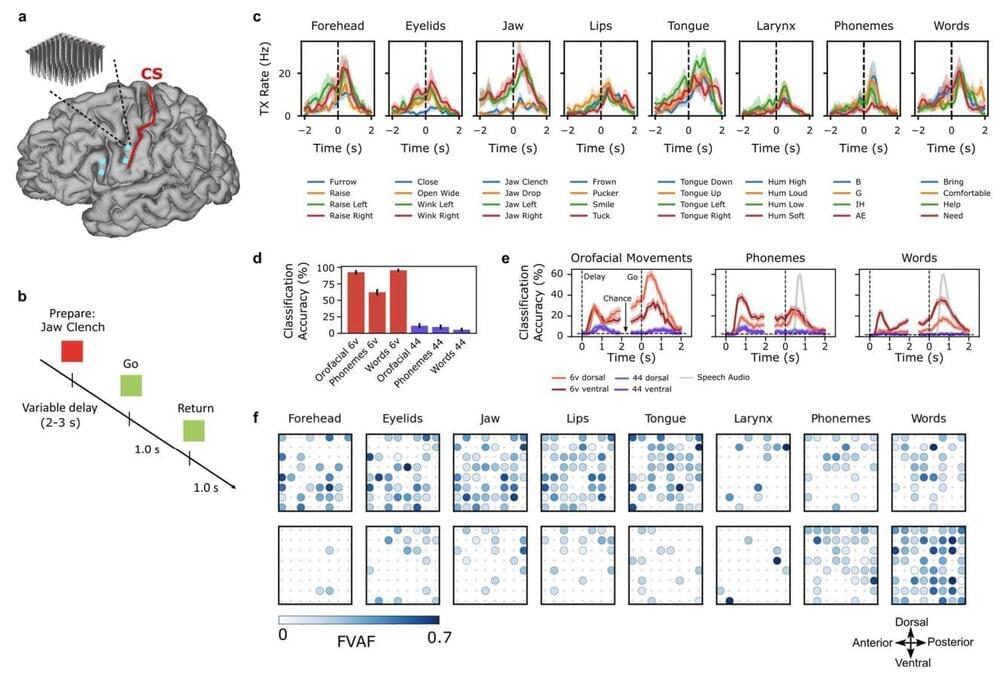
Artificial Intelligence has transformed how we live, work, and interact with technology. From voice assistants and chatbots to recommendation algorithms and self-driving cars, AI has suddenly become an integral part of our daily lives, just a few months after the release of ChatGPT, which kickstarted this revolution.
However, with the increasing prevalence of AI, a new phenomenon called “AI fatigue” has emerged. This fatigue stems from the overwhelming presence of AI in various aspects of our lives, raising concerns about privacy, autonomy, and even the displacement of human workers.
AI fatigue refers to the weariness, frustration, or anxiety experienced by individuals due to the overreliance on AI technologies. While AI offers numerous benefits, such as increased efficiency, improved decision-making, and enhanced user experiences, it also presents certain drawbacks. Excessive dependence on AI can lead to a loss of human agency, diminishing trust in technology, and a feeling of disconnection from the decision-making process.