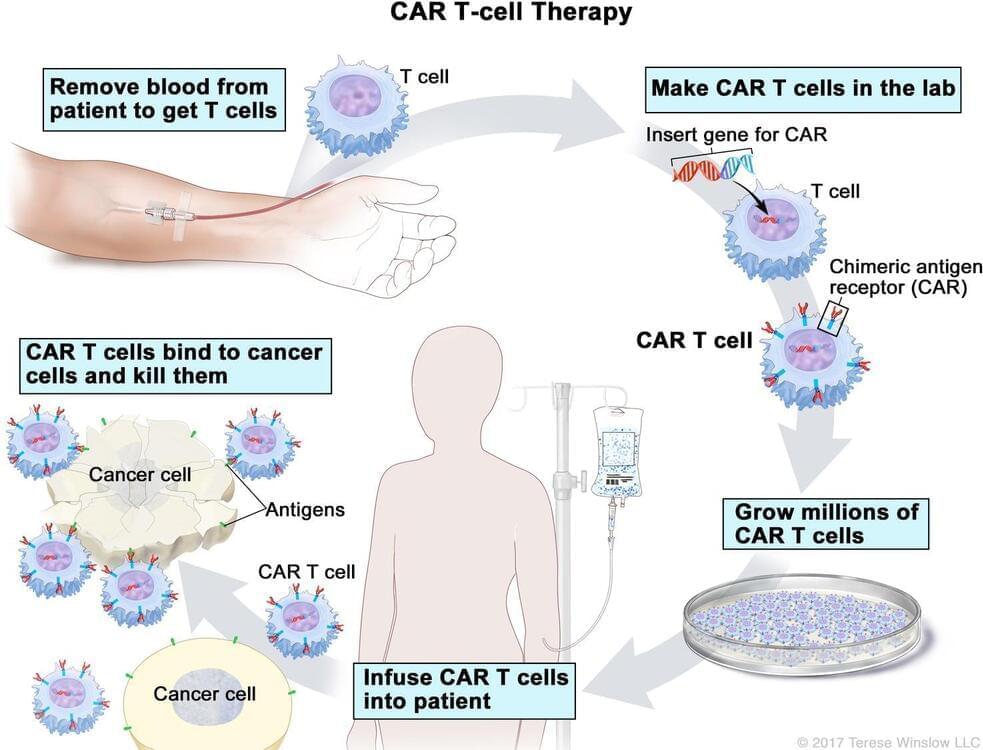
T-cell transfer therapy is a type of immunotherapy that makes your own immune cells better able to attack cancer. There are two main types of T-cell transfer therapy: tumor-infiltrating lymphocytes (or TIL) therapy and CAR T-cell therapy. Both involve collecting your own immune cells, growing large numbers of these cells in the lab, and then giving the cells back to you through a needle in your vein. T-cell transfer therapy is also called adoptive cell therapy, adoptive immunotherapy, and immune cell therapy.
The process of growing your T cells in the lab can take 2 to 8 weeks. During this time, you may have treatment with chemotherapy and, maybe, radiation therapy to get rid of other immune cells. Reducing your immune cells helps the transferred T cells to be more effective. After these treatments, the T cells that were grown in the lab will be given back to you via a needle in your vein.