Pharma startup Orsight sets its sights on preserving vision in patients with AMD.




Here’s a new Forbes review by world leading futurist Tracey Follows on the book: Transhuman Citizen:
What does Transhumanism, Ayn Rand and the U.S. Presidential election have in common? They are the connecting themes in a new book by Ben Murnane entitled, “Transhuman Citizen”
The book tells the story of Zoltan Istvan, a one-time U.S. Presidential candidate, who drove a coffin-shaped bus around the U.S. attempting to persuade the public that death is not inevitable and that transhumanism is a political as much as a scientific solution to the troubles of the 21st Century.
The book deals with what lead up to that Presidential campaign, the campaign itself, and what has happened since.
It starts with an explanation of how the author came to settle on his subject of Zoltan Istvan Gyurko, and the radical changes he wants to see in society. It links the author’s interest to his own personal circumstances. Murnane has a rare genetic disease, Fanconi anaemia, and became the first person in Ireland to have a novel form of bone marrow transplant. Having benefited from advanced medical technologies, he went on to write a book about living with the illness. Murnane also has interest in Ayn Rand, having completed a PhD in Rand and Posthumanism.

A new study indicates a link between sleep apnea and increased memory or thinking problems, based on self-reported data from over 4,000 participants.
People who experience sleep apnea may be more likely to also have memory or thinking problems, according to a preliminary study that will be presented at the American Academy of Neurology’s 76th Annual Meeting taking place April 13–18, 2024, in person in Denver and online. The study shows a positive association but did not determine whether sleep apnea causes cognitive decline.
Sleep apnea is when people stop and restart breathing repeatedly during sleep which can lower oxygen levels in the blood. Symptoms include snorting, gasping, and breathing pauses. People with the disorder may also experience morning headaches or have trouble focusing on tasks.

Leafhoppers, a common backyard insect, secrete and coat themselves in tiny mysterious particles that could provide both the inspiration and the instructions for next-generation technology, according to a new study led by Penn State researchers. In a first, the team precisely replicated the complex geometry of these particles, called brochosomes, and elucidated a better understanding of how they absorb both visible and ultraviolet light.
This could allow the development of bioinspired optical materials with possible applications ranging from invisible cloaking devices to coatings to more efficiently harvest solar energy, said Tak-Sing Wong, professor of mechanical engineering and biomedical engineering. Wong led the study, which was published today (March 18) in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).

Can anyone think of a good use for this protein in our cells in the future? Perhaps in space travel? 😁
Freeze ‘em, heat ‘em, blast them into empty space; with survival skills unlike any other organism on the planet, those hardy critters known as tardigrades will only come back for more.
While it’s clear their ability to withstand stress is in part due to their ability to turn their insides into gel, the mechanisms behind this act of metabolic preservation haven’t yet been made clear.
A new study led by researchers from the University of Wyoming found that expressing key tardigrade proteins in human cells slowed metabolism, providing critical insights into how these virtually indestructible invertebrates can survive under the most extreme conditions.

An international research team led by RMIT University has designed and manufactured a virus-killing surface that could help control disease spread in hospitals, labs, and other high-risk environments. The surface made of silicon is covered in tiny nanospikes that skewer viruses on contact.
Lab tests with the hPIV-3 virus – which causes bronchitis, pneumonia, and croup – showed 96% of the viruses were either ripped apart or damaged to the point where they could no longer replicate to cause infection. These impressive results, featured on the cover of top nanoscience journal ACS Nano, show the material’s promise for helping control the transmission of potentially dangerous biological material in laboratories and healthcare environments.
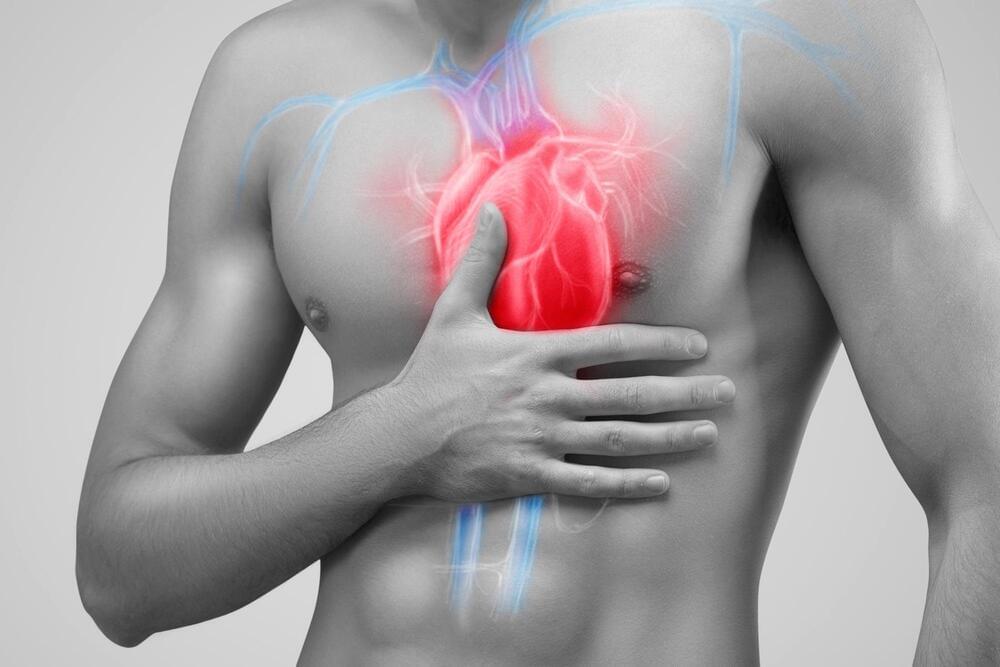
A new analysis involving over 13,000 people has found changes to blood vessels in the brain that can increase the risk of stroke and dementia are common in people with a range of heart conditions, regardless of whether they have experienced a stroke.
The new research, published in Neurology, the medical journal of the American Academy of Neurology, is the most comprehensive systematic review of ‘hidden’ brain changes in people with a range of heart conditions to date.
Lead author Dr Zien Zhou from The George Institute for Global Health said that identifying these changes could play an important role in choosing treatments for these patients.
Tryptophan is an essential amino acid, which means that it cannot be produced by the body but must be included as part of our diet. People with chronic bowel inflammation consume significantly more tryptophan than healthy people, as shown by previous research that involved members of the Cluster of Excellence “Precision Medicine in Chronic Inflammation” (PMI) at Kiel University.