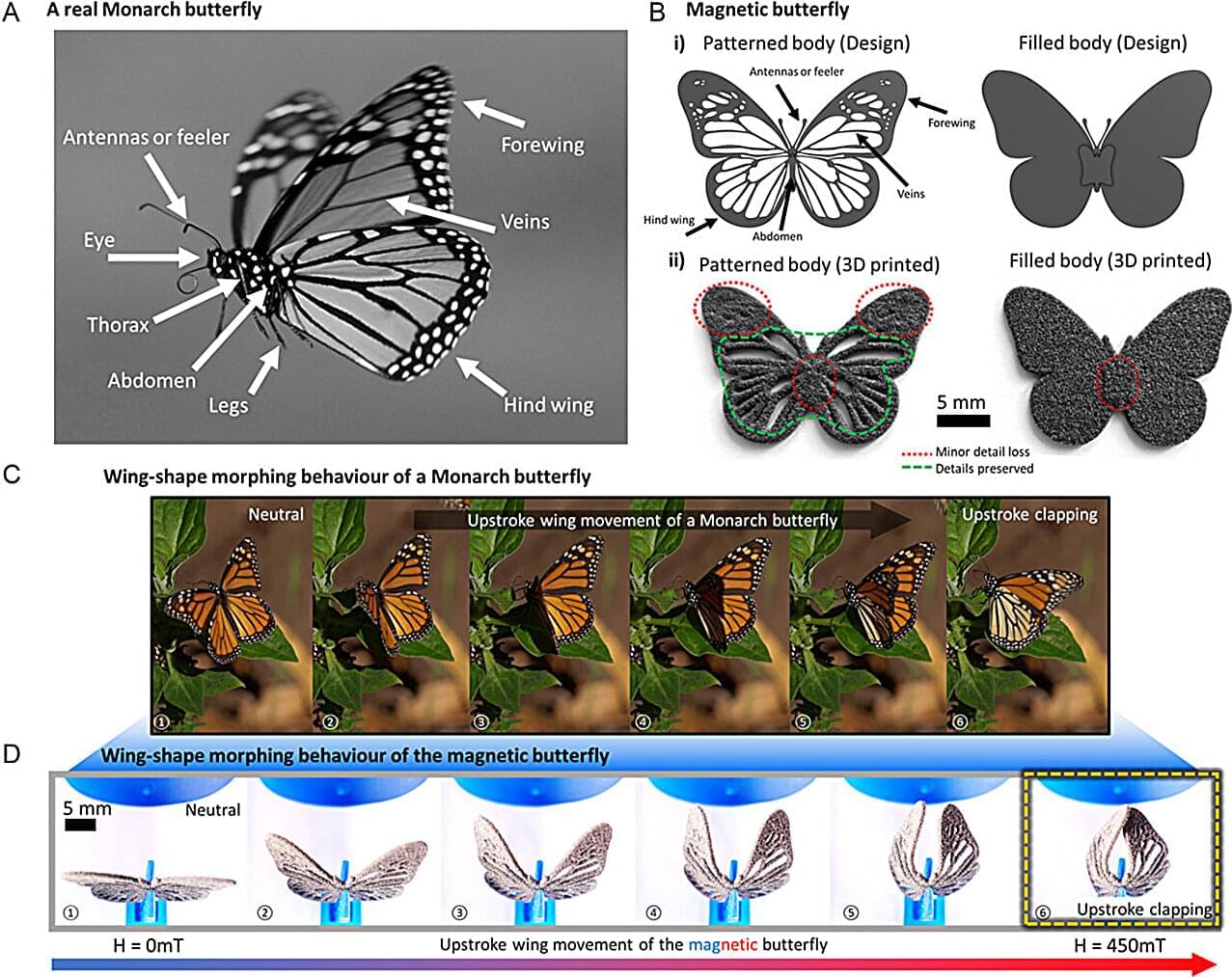
When 3D printing was first introduced in 1985, it marked a major turning point for the manufacturing industry. In addition to being cheaper than traditional manufacturing technologies, it also promised the ability to customize designs and make prototypes on demand. While its technology is still considered relatively new, there has been an accelerating demand for 3D printing methods across sectors in the past decade, ranging from aerospace and defense to medicine.
Yet, Associate Professor Pablo Valdivia y Alvarado from the Singapore University of Technology and Design (SUTD) believes that there are still ways to go before 3D printing can achieve its full potential. In traditional 3D printing, a nozzle is used to print the material layer by layer, and the path that the nozzle takes is known as the toolpath.
However, layer-by-layer printing is incompatible for use with materials like silicone, epoxies, and urethanes that are slow-curing and take more time to harden. These types of materials are often used to create soft mechanical metamaterials which, in turn, are used for lightweight, nature-inspired structures, such as lattices and web structures. Deposition-based processes in 3D printing, such as direct ink writing, would be able to work with these materials to create such structures, but these suffer from non-optimized toolpaths.