Researchers from the NIHR Moorfields Biomedical Research Centre and University College London have found that gene therapy improved visual acuity and preserved retinal structure in young children with AIPL1-associated severe retinal dystrophy. This is the first human trial of gene supplementation therapy targeting this condition.
Retinal dystrophy caused by biallelic variants in the AIPL1 gene leads to severe visual impairment from birth, with progressive degeneration and limited treatment options. Previous studies of early-onset rod-cone dystrophies, including AIPL1-related forms, highlighted a critical window for intervention during early childhood, when some photoreceptor structure remains intact. Prior research using Aipl1-deficient mouse models and human retinal organoids demonstrated partial restoration of photoreceptor function through gene therapy.
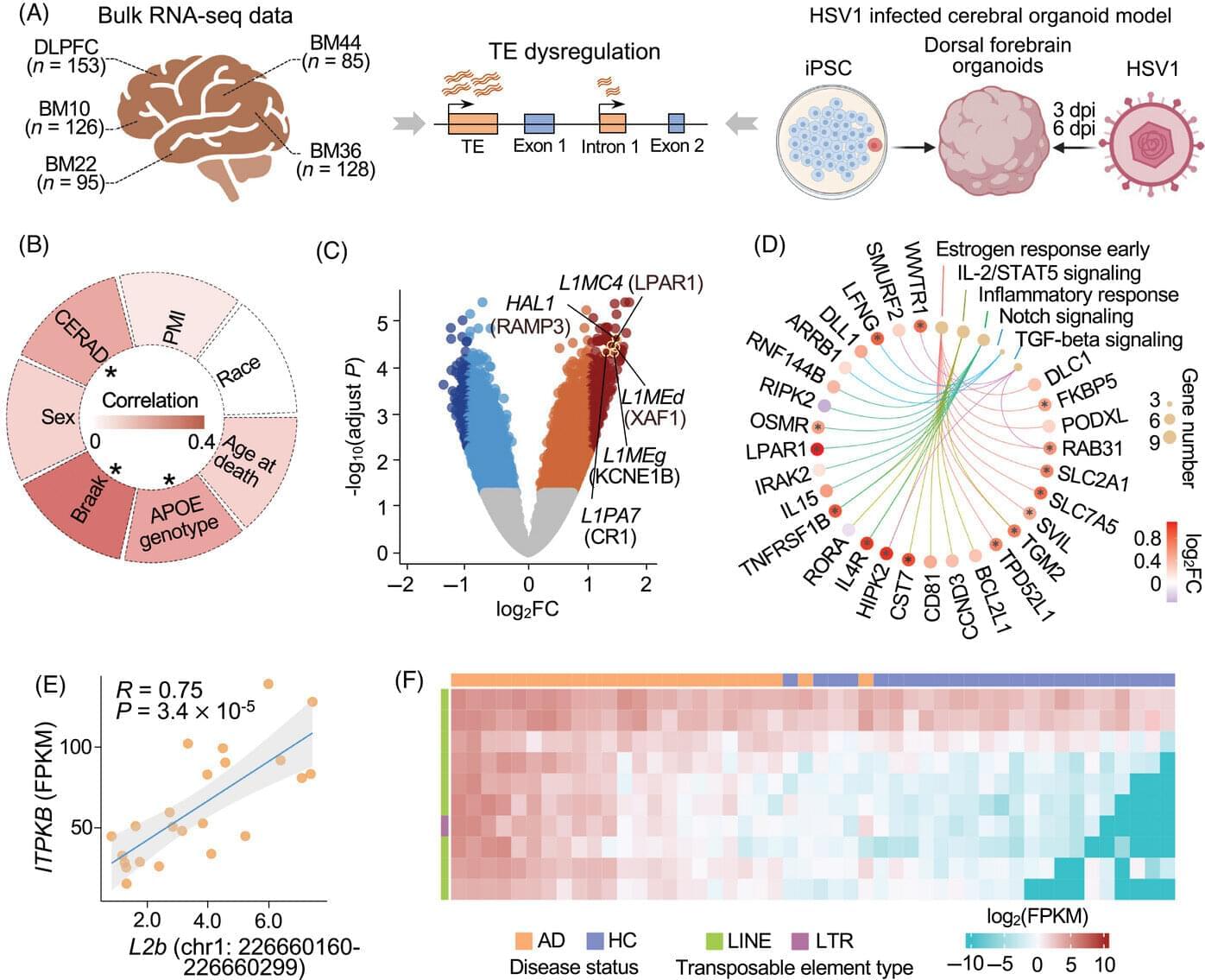
In the study, “Gene therapy in children with AIPL1-associated severe retinal dystrophy: an open-label, first-in-human interventional study,” published in The Lancet, researchers administered a single subretinal injection of a recombinant adeno-associated viral vector (rAAV8.hRKp. AIPL1) carrying the AIPL1 gene to one eye of each child to assess the safety and efficacy of gene supplementation therapy in improving visual function and preserving retinal structure.