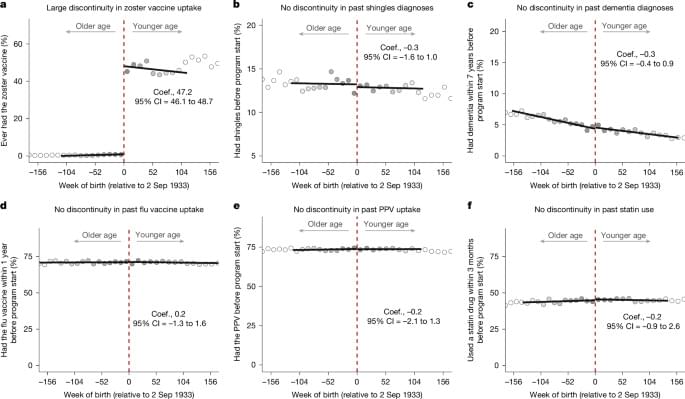
Using a natural experiment that avoids common bias concerns, this study finds that the live-attenuated shingles vaccine reduced the probability of a new dementia diagnosis within a follow-up period of 7 years by approximately one-fifth.

Satya Nadella, CEO of Microsoft, shares the groundbreaking potential of AI Copilot — a powerful tool that’s transforming how we work. From streamlining everyday tasks to revolutionizing healthcare workflows, AI Copilot is designed to seamlessly integrate with the tools we already use, like Teams, Word, and Excel.
Satya Nadella explains how AI Copilot is helping doctors prepare for high-stakes meetings, automatically generating agendas, summaries, and even PowerPoint presentations. Plus, see how it empowers professionals to gather the latest insights, collaborate with teams, and create smarter workflows with ease.
Thank You for watching! Do not forget to Like | Comment | Share.
About the channel.
Watch us for the best news and views on business, stock markets, crypto currencies, consumer technology, the world of real estate, bullion, automobiles, start-ups and unicorns and personal finance. Business Today TV will also bring you all you need to know about mutual funds, insurance, loans and pension plans among others.
Follow us at:

Brigham and Women’s Hospital-led research reports no significant long-term benefit of cocoa flavanol supplementation in preventing age-related macular degeneration (AMD). The paper is published in the journal JAMA Ophthalmology.
AMD is a progressive retinal disease and the most common cause of severe vision loss in adults over age 50. AMD damages the macula, the central part of the retina responsible for sharp, detailed vision. While peripheral sight is typically preserved, central vision loss can impair reading, driving, facial recognition, and other quality of life tasks. Abnormalities of blood flow in the eye are associated with the occurrence of AMD.
Cocoa flavanols are a group of naturally occurring plant compounds classified as flavonoids, found primarily in the cocoa bean. These bioactive compounds have been studied for their vascular effects, including improved endothelial function and enhanced nitric oxide production, which contribute to vasodilation and circulatory health. Previous trials have shown that moderate intake of cocoa flavanols may lower blood pressure, improve lipid profiles, and reduce markers of inflammation, suggesting a role in mitigating cardiovascular and related vascular conditions.

We’re exploring the frontiers of AGI, prioritizing readiness, proactive risk assessment, and collaboration with the wider AI community.
Artificial general intelligence (AGI), AI that’s at least as capable as humans at most cognitive tasks, could be here within the coming years.
Integrated with agentic capabilities, AGI could supercharge AI to understand, reason, plan, and execute actions autonomously. Such technological advancement will provide society with invaluable tools to address critical global challenges, including drug discovery, economic growth and climate change.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
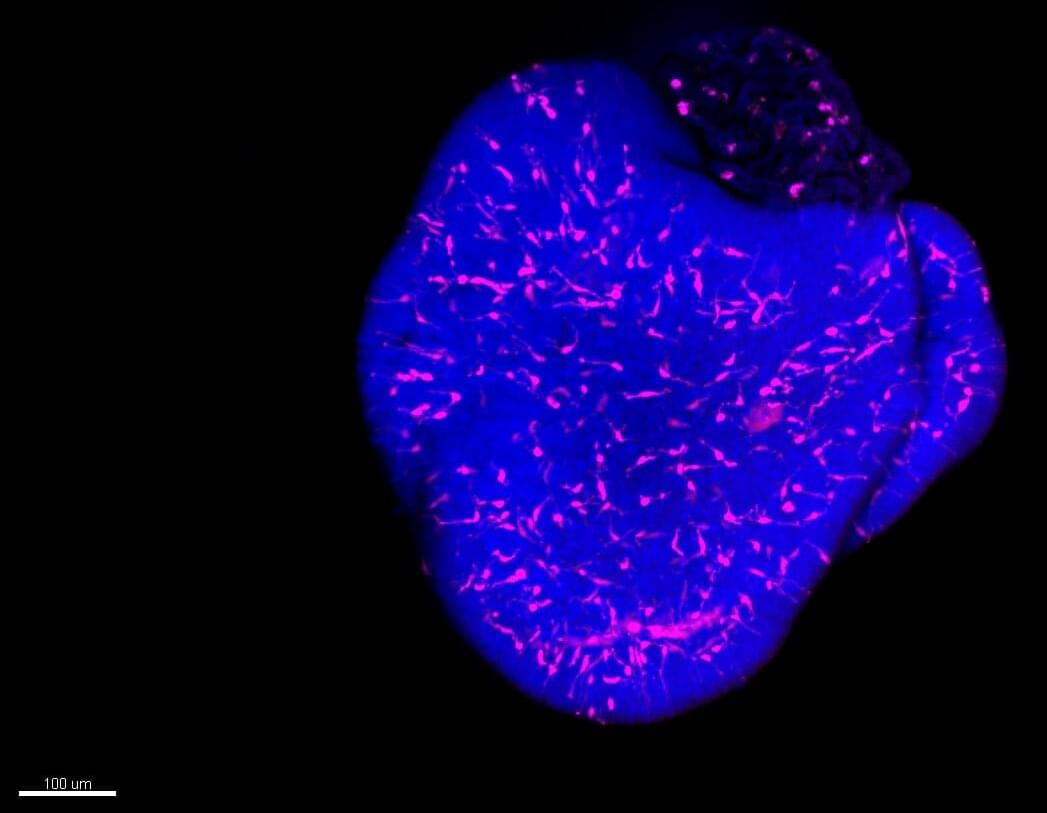
Organoids have revolutionized science and medicine, providing platforms for disease modeling, drug testing, and understanding developmental processes. While not exact replicas of human organs, they offer significant insights.
The Siegert group at the Institute of Science and Technology Austria (ISTA) presents a new organoid model that reveals details of the developing nervous system’s response to viral infections, such as Rubella. This model could influence pharmaceutical testing, particularly benefiting drug safety for pregnant women.
Microglia are special cells in the human brain. Like a diligent ranger overseeing a forest and dealing with infestations and wildfires, microglia scan the brain environment for germs and initiate an anti-inflammatory response to remove them. They also monitor the quantity of neurons (nerve cells) and their connections to ensure optimal brain function in adulthood.
Nearly 16 million American adults have been diagnosed with attention deficit hyperactivity disorder (ADHD), but evidence suggests that more than 30% of them don’t respond well to stimulant medications like Ritalin and Adderall.
A new clinical trial provides a surprising explanation for why this may be the case: There are individual differences in how our brain circuits are wired, including the chemical circuits responsible for memory and concentration, according to a new study co-led by the University of Maryland School of Medicine (UMSOM) and performed at the National Institutes of Health (NIH) Clinical Center.
Our brain cells have different types of chemical receptors that work together to produce optimal performance of brain function. Differences in the balance of these receptors can help explain who is likely to benefit from Ritalin and other stimulant medications. That is the finding of the new research published in the Proceedings of the National Academy of Sciences.
Lariocidin was efficient against strains of E. coli, including drug-resistant ones, according to the new study.
Researchers say they have discovered a new class of antibiotics that could treat drug-resistant bacteria, the first to reach the market in nearly three decades.
The new molecule, called lariocidin, works by targeting a part of a bacteria’s cell called the ribosome and can disrupt the cell’s functions.