Reduced exposure to arsenic in drinking water was linked to fewer deaths from chronic disease, including cancer and cardiovascular disease.


Reducing calorie intake helps cancer-fighting immune cells do their jobs more effectively, reports a study by Van Andel Institute scientists and collaborators. The findings lay the groundwork for developing dietary strategies to boost the effects of a powerful class of cancer immunotherapies.
“Growing evidence suggests dietary restriction has anti-cancer effects but the ‘why and how’ are not well understood. Our new study reveals one way this relationship may work: by providing T cells, the soldiers of the immune system, with the right mix of nutrients to more effectively fight cancer,” said Russell Jones, Ph.D., chair of VAI’s Department of Metabolism and Nutritional Programming and corresponding author of the study.
“Additional research is needed but we are hopeful these insights can inform evidence-based dietary guidelines to improve the effectiveness of immune-based cancer treatments.”
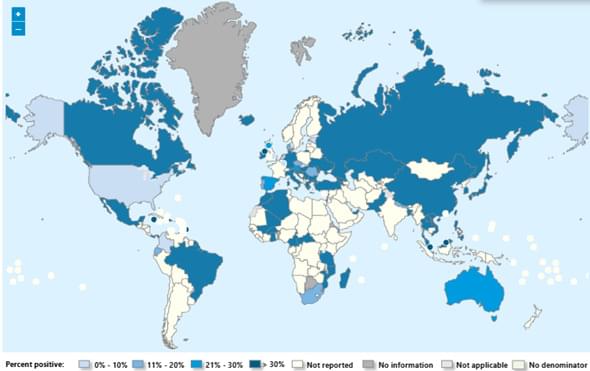
Seasonal influenza activity has increased globally in recent months, and influenza A(H3N2) viruses are predominant. This rise coincides with the onset of winter in the northern hemisphere. Epidemics and outbreaks of seasonal influenza and other circulating respiratory viruses can place significant pressure on healthcare systems. Although global activity remains within expected seasonal ranges, early increases and higher activity than typical at this time of year have been observed in some regions. Seasonal influenza could place significant pressure on healthcare systems even in non-temperate countries. Genetically drifted influenza A(H3N2) viruses, known as subclade K viruses, have been detected in many countries. While data on how well the vaccine works against clinical disease this season are still limited, vaccination is still expected to protect against severe illness and remains one of the most effective public health measures.
Surveillance
Due to the constantly evolving nature of influenza viruses, WHO continues to stress the importance of year-round global surveillance to detect and monitor virological, epidemiological and clinical changes associated with emerging or circulating influenza viruses that may affect human health and timely virus sharing for risk assessment. Countries are encouraged to remain vigilant to the threat of influenza viruses and review any unusual epidemiological patterns.
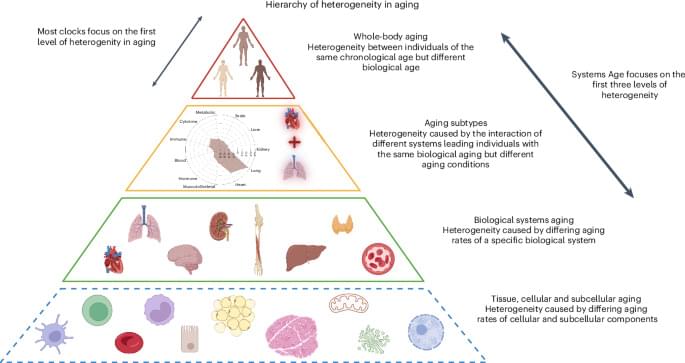
We developed a single blood-based methylation test that estimates biological aging across 11 physiological systems. This multisystem measure predicts mortality and health outcomes more precisely than existing epigenetic clocks, and reveals distinct aging patterns that could guide personalized gerotherapeutic and geroprotective interventions.

(Cell Reports 23, 112–126; April 3, 2018)
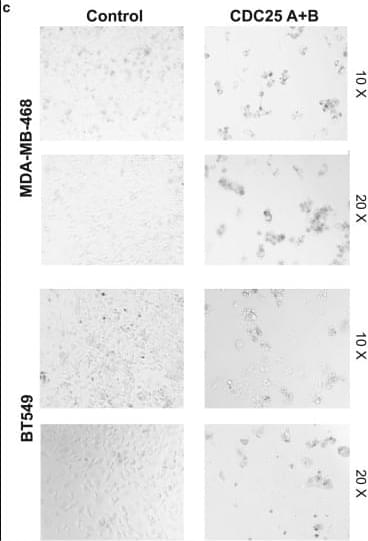
In the originally published version of this article on April 3, 2018, Figure 5C contained two representative images for each treatment condition. One image was inadvertently duplicated in the panel depicting the combined knockdown of CDC25a and CDC25b. The authors have reviewed the original experimental data and provided corrected images. The revised Figure 5C now accurately presents the results for MDA-MB-468 and BT549 cells following double transfection with CDC25a and CDC25b RNAi. The error and correction thereof do not affect the conclusions of the original manuscript. The authors sincerely apologize for any inconvenience or confusion that may have been caused.
Australians in their 30s and 40s are facing an alarming surge in cancer diagnoses and researchers are scrambling to understand why.
From bowel and breast to liver and kidney, aggressive cancers are hitting younger people; they’re often detected late, with devastating outcomes.
Dr Norman Swan investigates what’s behind the change.
Could it be ultra-processed foods, stress, or exposures dating back to childhood, even pregnancy?
He meets those grappling with a diagnosis and searching for answers.
Generation Cancer asks what can be done to curb the rise, and are we ready?
Spanish researchers have created a powerful new open-source tool that helps uncover the hidden genetic networks driving cancer. Called RNACOREX, the software can analyze thousands of molecular interactions at once, revealing how genes communicate inside tumors and how those signals relate to patient survival. Tested across 13 different cancer types using international data, the tool matches the predictive power of advanced AI systems—while offering something rare in modern analytics: clear, interpretable explanations that help scientists understand why tumors behave the way they do.

Medicines used for cancer treatment often cause serious side effects by damaging normal cells due to nonspecific diffusion. To address this issue, we previously developed an optical method to induce apoptotic cell death via intracellular pH alkalinization using the outward proton pump rhodopsin, Archaerhodopsin-3 (AR3) in various noncancer model cells in vitro and in vivo. In this study, we applied this method to cancer cells and tumors to evaluate its potential as an anticancer therapeutic strategy. First, we confirmed that AR3-expressing murine cancer cell lines (MC38, B16F10) showed apoptotic cell death upon green light irradiation, as indicated by increased levels of cell death and apoptosis-related markers. Next, we established stable AR3-expressing MC38 and B16F10 cells by using viral vectors. When these AR3-expressing cells were subcutaneously transplanted into C57BL/6 mice, the resulting tumors initially grew at a rate comparable to that of control tumors lacking AR3 expression or light stimulation. However, upon green light irradiation, AR3-expressing tumors exhibited either a marked reduction in size or significantly suppressed growth, accompanied by the induction of apoptosis signals and decreased proliferation signals. These results demonstrate that AR3-mediated cell death has potent antitumor effects both in vitro and in vivo. This optical method thus holds promise as a novel cancer therapy with potentially reduced side effects.
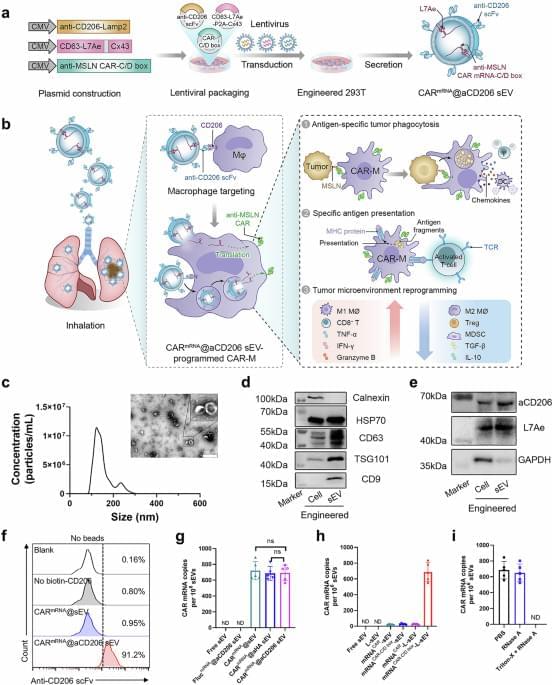
Chimeric antigen receptor (CAR) macrophages may represent a promising anti-cancer immune therapy strategy due to their favourable biological properties but in vitro manipulation and targeting to specific organs could be challenging. Here authors use inhalable small extracellular vesicles to deliver the CAR mRNA construct to macrophages in the lung and show that the thus in situ generated CAR macrophages successfully combat lung metastasis and cancer recurrence in a mouse model.