NASA crew and ground-based scientists are sending blood cells to the International Space Station on November 4 to learn why astronauts have a higher risk of blood clots.



Dr. Abba Zubair, MD: “Our hope is to study these space-grown cells to improve treatment for age-related conditions such as stroke, dementia, neurodegenerative diseases and cancer.”
What can microgravity teach us about stem cell growth? This is what a recent study published in NPJ Microgravity hopes to address as a pair of researchers from the Mayo Clinic investigated past research regarding the growth properties of stem cells, specifically regeneration, differentiation, and cell proliferation in microgravity and whether the stem cells can maintain these properties after returning to Earth. This study holds the potential to help researchers better understand how stem cell growth in microgravity can be transitioned into medical applications, including tissue growth for disease modeling.
“The goal of almost all space flight in which stem cells are studied is to enhance growth of large amounts of safe and high-quality clinical-grade stem cells with minimal cell differentiation,” said Dr. Abba Zubair, MD, who is a faculty at the Mayo Clinic and the sole co-author on the study. “Our hope is to study these space-grown cells to improve treatment for age-related conditions such as stroke, dementia, neurodegenerative diseases and cancer.”
For the study, the researchers examined past research that launched stem cell cultures to the International Space Station (ISS) to have astronauts onboard evaluate the stem cells’ growth patterns and behavior under microgravity conditions. Dr. Zunair has launched stem cells to the ISS on three occasions and the various types of stem cells examined on the ISS in previous research include mesenchymal stem cells, hematopoietic stem cells, cardiovascular progenitor stem cells, and neural stem cells.
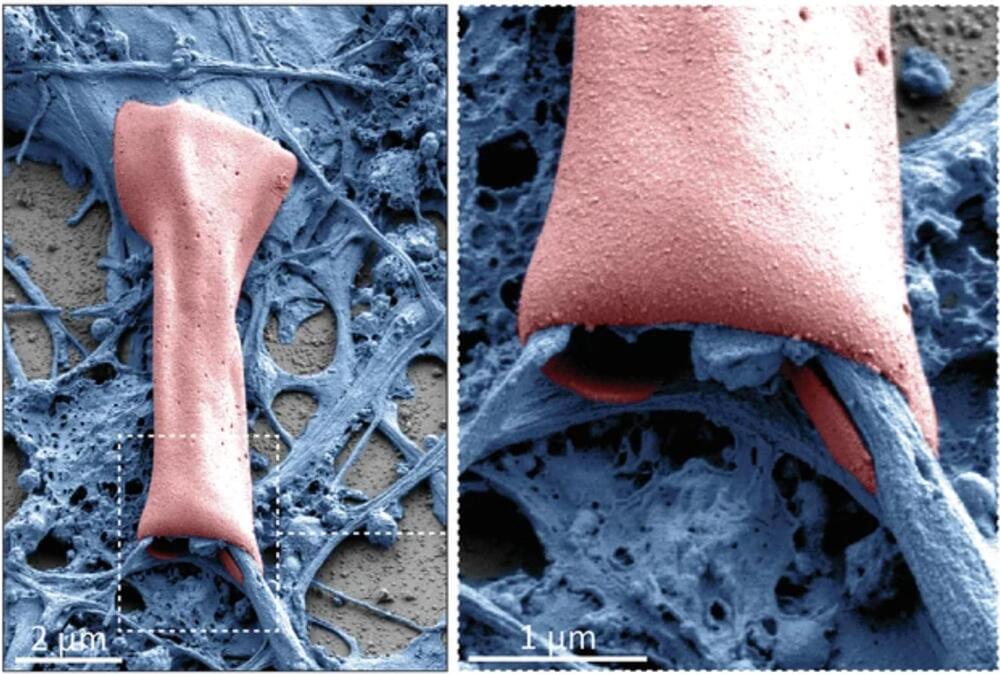
A study led by Umeå University, Sweden, presents new insights into how stem cells develop and transition into specialized cells. The discovery can provide increased understanding of how cells divide and grow uncontrollably so that cancer develops.
“The discovery opens a new track for future research into developing new and more effective treatments for certain cancers,” says Francesca Aguilo, associate professor at the Department of Molecular Biology at Umeå University and leader of the study in collaboration with various institutions including the University of Pavia, University of Texas Health Science Center at Houston, Universidad de Extremadura, and others.
All cells in the body arise from a single fertilized egg. From this single origin, various specialized cells with widely differing tasks evolve through a process called cellular differentiation. Although all cells share the same origin and share the same genetic information, specialized cells use the information in different ways to perform different functions. This process is regulated by genetic and epigenetic mechanisms.
In a world where choices seem endless, could it be that our ‘free will’ is nothing more than an illusion?
When it comes to things like choosing a morning run over an extra hour of sleep, opting for an apple instead of that enticing pint of ice cream, or quitting your job on a whim…
…What’s truly guiding these decisions? Is it willpower, biology, environment, or perhaps a unique strength of character we’ve built over time?
Or… could it be something else entirely, something beyond our control?
Here’s where our guest, Dr. Robert Sapolsky — a renowned Professor of Biology, Neurology and Neurosurgery at Stanford University — offers us a slightly unsettling, yet eye-opening, perspective.
He suggests that every decision we make — from the podcasts we tune into, to judges making a case verdict, to choosing our life partner — isn’t shaped by any sort of conscious control or free will. Instead, he believes our actions are driven by factors beyond our grasp and influence.

Scientists in China have managed to revive brain activity in pigs nearly an hour after circulation ceased, thanks to the surprising involvement of the liver.
If translatable to humans, this finding could have significant implications for extending the critical window in which doctors can resuscitate patients following sudden cardiac arrest.
The research team, led by Dr. Xiaoshun He at Sun Yat-Sen University, experimented with the brains of 17 Tibetan minipigs to investigate how the liver might influence brain recovery.

In a rat experiment, researchers publishing in Aging Cell have found that senescent cells and SASP factors are key in regenerating knee cartilage.
Not always negative
Cellular senescence is widely known to have negative effects, to the point that it is one of the hallmarks of aging. In fact, rather than protecting cartilage, cellular senescence has been reported to damage it in the progression of osteoarthritis [1]. However, the idea that senescence is beneficial for regeneration is not a new concept [2], and it has been found to assist wound healing in mice [3]. Understanding everything involved in this complex relationship is not easy, and one of the factors appears to be windows of time [4].

Researchers tracked the health of nearly one thousand mice on a variety of diets to see if these diets would extend the mice’s lifespan. The study was designed to ensure that each mouse was genetically distinct, which allowed the team to better represent the genetic diversity of the human population. By doing so, the results are made more clinically relevant, elevating the study to one of the most significant investigations into aging and lifespan to date.
For nearly a century, laboratory studies have shown consistent results: eat less food, or eat less often, and an animal will live longer. But scientists have struggled to understand why these kinds of restrictive diets work to extend lifespan, and how to best implement them in humans. Now, in a long-awaited study to appear in the Oct. 9 issue of Nature, scientists at The Jackson Laboratory (JAX) and collaborators tracked the health of nearly one thousand mice on a variety of diets to make new inroads into these questions.
The study was designed to ensure that each mouse was genetically distinct, which allowed the team to better represent the genetic diversity of the human population. By doing so, the results are made more clinically relevant, elevating the study to one of the most significant investigations into aging and lifespan to date.