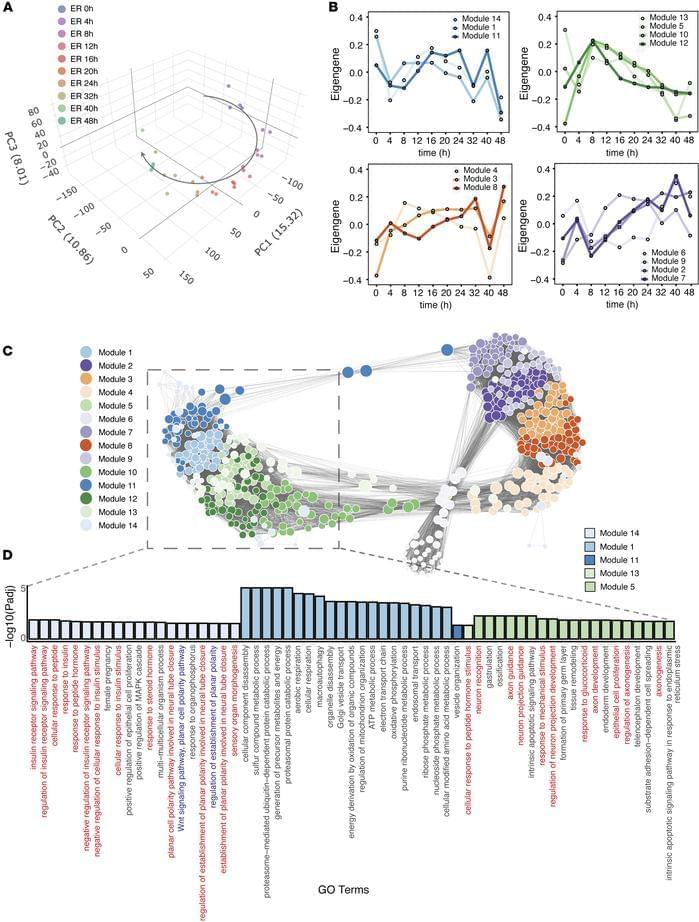
Megha Padi & team now show pyrvinium pamoate has antitumor activity through multiple pathways in pre-clinical models:
The figure shows tumor tissue from control mice or those treated with pyrvinium.
1University of Arizona Cancer Center, Tucson, Arizona, USA.
2Department of Molecular and Cellular Biology, University of Arizona, Tucson, Arizona, USA.
3Department of Pharmacology and Toxicology, The University of Arizona R. Ken Coit College of Pharmacy, Skaggs Pharmaceutical Sciences Center, Tucson, Arizona, USA.