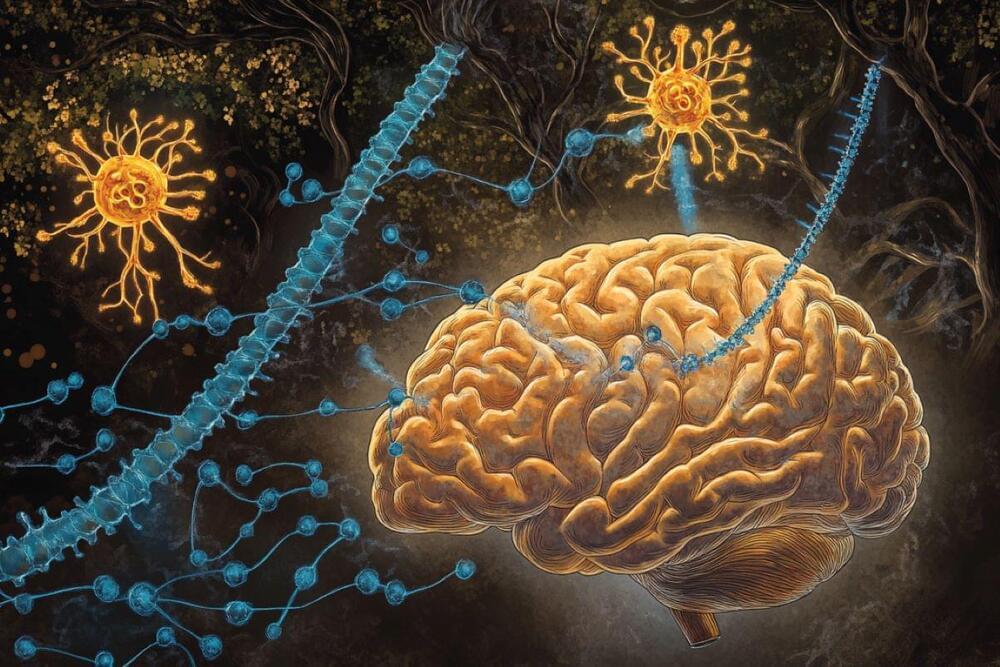
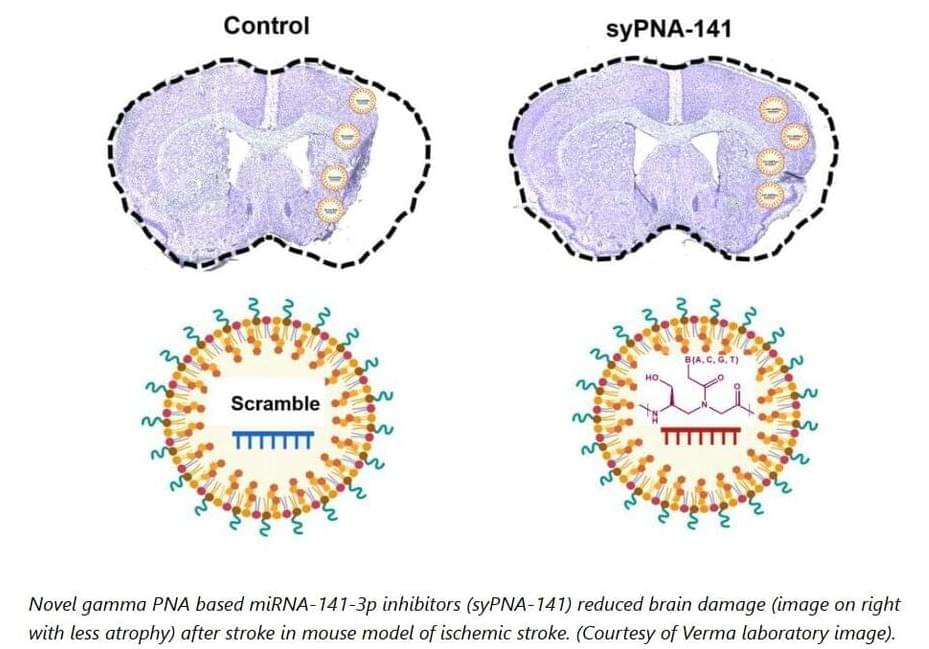
It’s been more than three decades, but still there are only two treatments for a stroke: either rapid use of a clot-busting medication called tPA or surgical removal of a clot from the brain with mechanical thrombectomy. However, only 5% to 13% percent of stroke cases are actually eligible for these interventions.
“We need to be persistent with our research to find a new therapy for stroke,” says Rajkumar Verma, M.Pharm., Ph.D., assistant professor, Department of Neuroscience at UConn School of Medicine working in cross-campus collaboration with Professor Raman Bahal Ph.D. of the Department of Pharmaceutical Sciences in the UConn School of Pharmacy. “Stroke research is hard and challenging to do. But without trying we won’t make progress. We need to keep trying. UConn is determined to keep trying.”
In addition to being life-threatening, stroke is the major cause of long-term disability worldwide.