A new study reveals that mitochondrial dysfunction plays a central role in the loss of Purkinje neurons in the cerebellum, contributing to motor impairments in people with multiple sclerosis (MS).

The brain is constantly mapping the external world like a GPS, even when we don’t know about it. This activity comes in the form of tiny electrical signals sent between neurons—specialized cells that communicate with one another to help us think, move, remember and feel. These signals often follow rhythmic patterns known as brain waves, such as slower theta waves and faster gamma waves, which help organize how the brain processes information.
Understanding how individual neurons respond to these rhythms is key to unlocking how the brain functions related to navigation in real time—and how it may be affected in disease.
A new study by Florida Atlantic University and collaborators from Erasmus Medical Center, Rotterdam, Netherlands, and the University of Amsterdam, Netherlands, has uncovered a surprising ability of brain cells in the hippocampus to process and encode and respond to information from multiple brain rhythms at once.
Huntington’s disease has long defied attempts to rescue suffering neurons. A new study in Cell Reports shows that transplanting healthy human glial progenitor cells into the brains of adult animal models of the disease not only slowed motor and cognitive decline but also extended lifespan. These findings shift our understanding of Huntington’s pathology and open a potential path to cell-based therapies in adults already showing symptoms.
“Glia are essential caretakers of neurons,” said Steve Goldman, MD, Ph.D., co-director of the University of Rochester Center for Translational Neuromedicine and lead author of the study.
“The restoration of healthy glial support—even after symptoms begin—could reset neuronal gene expression, stabilize synaptic function, and meaningfully delay disease progression. This study shifts the perspective on Huntington’s from a neuron-centric view to one that shows a critical role for glial pathology in driving synaptic dysfunction. It also tells us that the adult brain still has the capacity for repair when you target the right cells.”

Researchers at Karolinska Institutet and Lund University in Sweden have identified a new treatment strategy for neuroblastoma, an aggressive form of childhood cancer. By combining two antioxidant enzyme inhibitors, they have converted cancer cells in mice into healthy nerve cells.
The study, “Combined targeting of PRDX6 and GSTP1 as a potential differentiation strategy for neuroblastoma treatment,” is published in the journal Proceedings of the National Academy of Sciences.
Neuroblastoma is a type of childhood cancer that affects the nervous system and is the leading cause of cancer-related death in young children. Some patients have a good prognosis, but those with metastatic tumors often cannot be cured despite modern combinations of surgery, radiation, chemotherapy and immunotherapy.
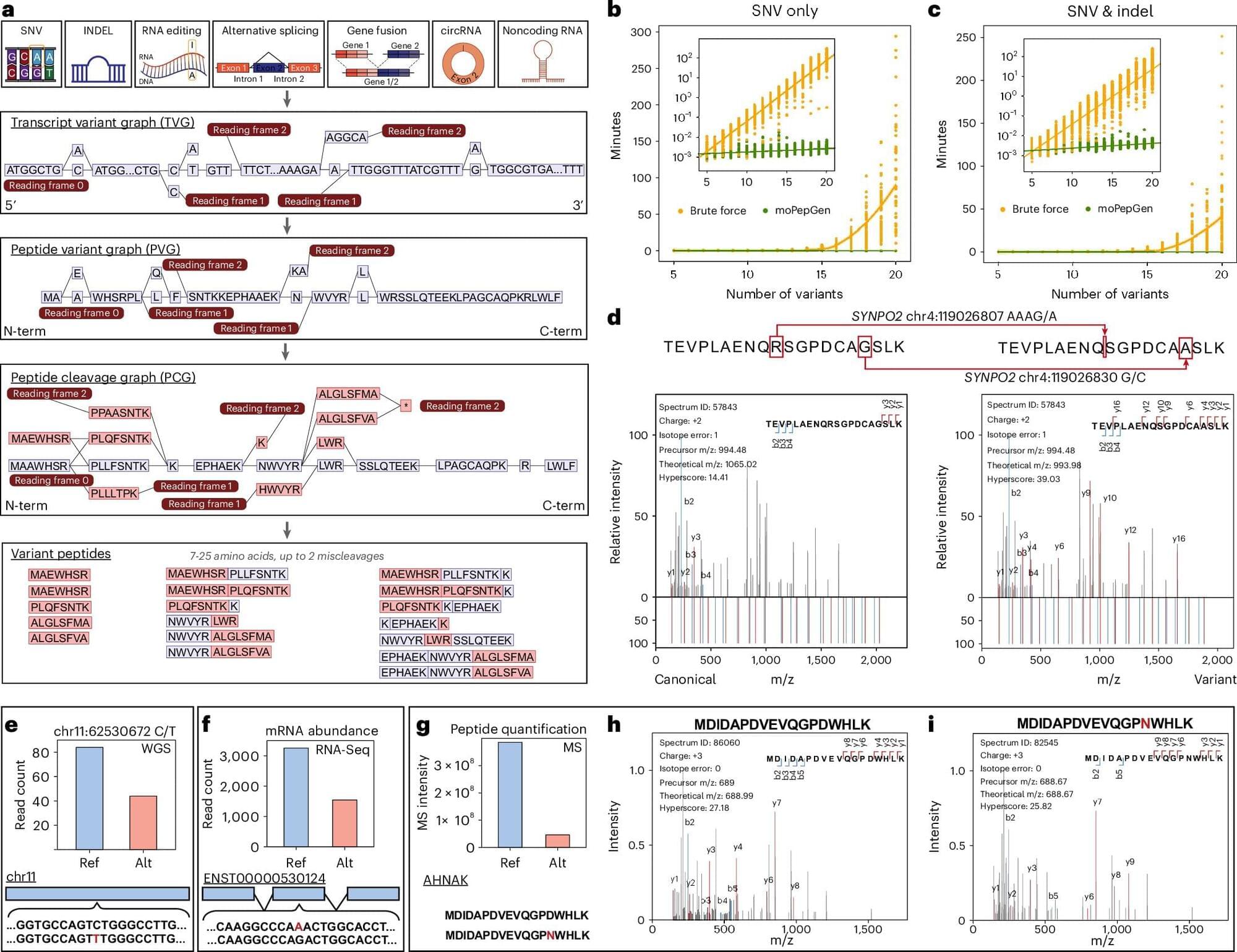
Scientists at UCLA and the University of Toronto have developed an advanced computational tool, called moPepGen, that helps identify previously invisible genetic mutations in proteins, unlocking new possibilities in cancer research and beyond.
The tool, described in Nature Biotechnology, will help understand how changes in our DNA affect proteins and ultimately contribute to cancer, neurodegenerative diseases, and other conditions. It provides a new way to create diagnostic tests and to find treatment targets previously invisible to researchers.
Proteogenomics combines the study of genomics and proteomics to provide a comprehensive molecular profile of diseases. However, a major challenge has been the inability to accurately detect variant peptides, limiting the ability to identify genetic mutations at the protein level. Existing proteomic tools often fail to capture the full diversity of protein variations.

Law enforcement authorities from six countries took down the Archetyp Market, an infamous darknet drug marketplace that has been operating since May 2020.
Archetyp Market sellers provided the market’s customers with access to high volumes of drugs, including cocaine, amphetamines, heroin, cannabis, MDMA, and synthetic opioids like fentanyl through more than 3,200 registered vendors and over 17,000 listings.
Over its five years of activity, the marketplace amassed over 612,000 users with a total transaction volume of over €250 million (approximately $289 million) in Monero cryptocurrency transactions.

PFOS, also known as “forever chemicals,” are synthetic compounds popular for several commercial applications, like making products resistant to stains, fire, grease, soil and water. They have been used in non-stick cookware, carpets, rugs, upholstered furniture, food packaging and firefighting foams deployed at airports and military airfields.
PFOS (perfluorooctane sulfonate or perfluorooctane sulfonic acid) are part of the larger class of forever chemicals called PFAS (per-and polyfluoroalkyl substances.) Both types have been linked to a variety of health issues, including liver disease, immune system malfunction, developmental issues and cancer.
Because of their widespread use, PFOS are found in soil, agricultural products and drinking water sources, presenting a health risk. Xiaoguang Meng and Christos Christodoulatos, professors at the Department of Civil, Environmental and Ocean Engineering at Stevens Institute of Technology, and Ph.D. student Meng Ji working in their lab, wanted to identify the most efficient way to remove these toxins from the water.

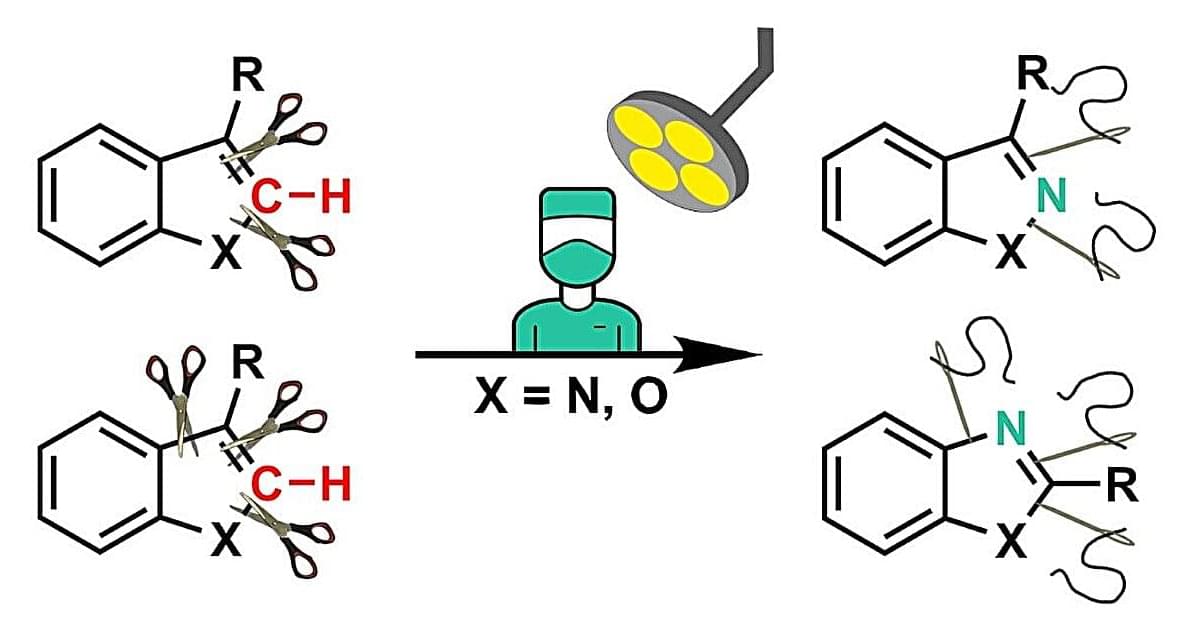
Skeletal editing is a modern approach to chemical synthesis. By making precise alterations at the atomic level, researchers are able to directly convert existing drug scaffolds into new, biologically relevant compounds.
A team led by chemist Prof Armido Studer from the University of Münster has developed a new strategy based on this technique to swap carbon atoms with nitrogen atoms (“C-to-N atom swapping”). The process functions within indole and benzofuran frameworks. These chemical structures, which contain two molecular rings consisting mainly of carbon, are common building blocks of pharmaceuticals and natural products.
“This technique expands the synthetic toolbox available for skeletal editing,” explains doctoral student Zhe Wang, who carried out most of the experimental work. It represents a step forward in the development of new molecules from established pharmacophores, i.e. the molecular components responsible for pharmacological effects.