Scientists decode cocoa DNA to create world’s most flavorful chocolate in lab.
Scientists are unlocking the secrets of cocoa fermentation by designing microbial communities that can recreate the flavors of chocolate.

Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links/Affiliates: Blood testing (where I get the majority of my labs): https://www.u…
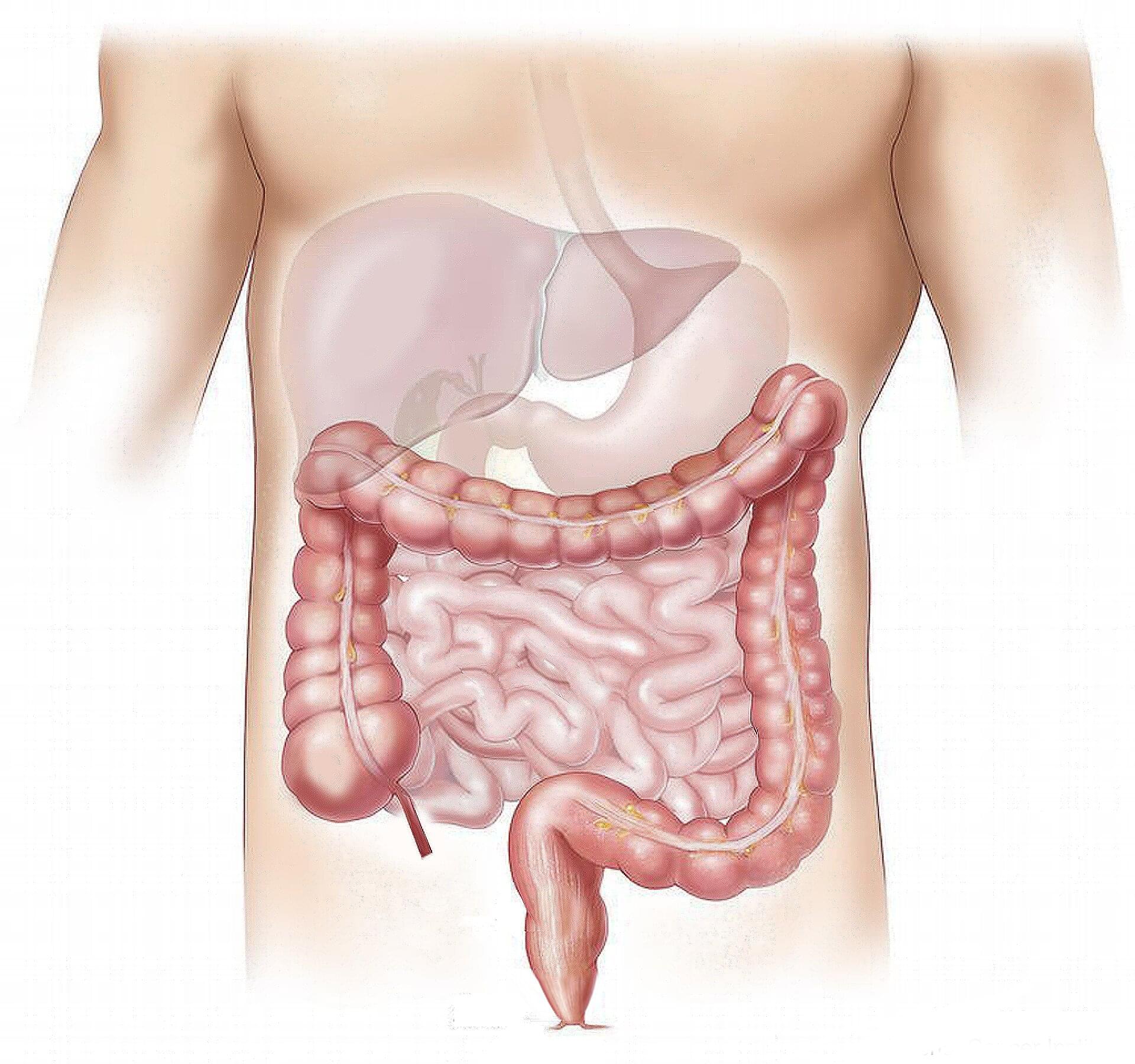
Cleveland Clinic researchers have discovered a connection between elevated blood levels of TMAO (trimethylamine N-oxide)—a byproduct of gut bacteria digestion of nutrients found in red meat and other animal products—and a higher risk of abdominal aortic aneurysms.
The findings, published in JAMA Cardiology, suggest that TMAO may play a role in the development, progression, and severity of this life-threatening condition, including faster rates of aneurysm expansion and greater risk for needing surgery.
“These results suggest targeting TMAO levels may help prevent and treat aneurysmal disease beyond surgery,” said lead author Scott Cameron, M.D., section head of Vascular Medicine at Cleveland Clinic.
You may not be aware that most of the medicines that have been approved for treatment are rooted in nature.
For example, the bark of willow trees has been called nature’s aspirin because it contains a chemical called salicin. The human body converts salicin into salicylic acid, which relieves pain and fights fevers.
New research by William Chain, associate professor in the University of Delaware’s Department of Chemistry and Biochemistry, and his lab, uses a molecule found in a tropical fruit to offer hope in the fight against liver-related cancers, one of the world’s top causes of cancer deaths.
Chemistry breakthrough provides pathway to low cost treatments.
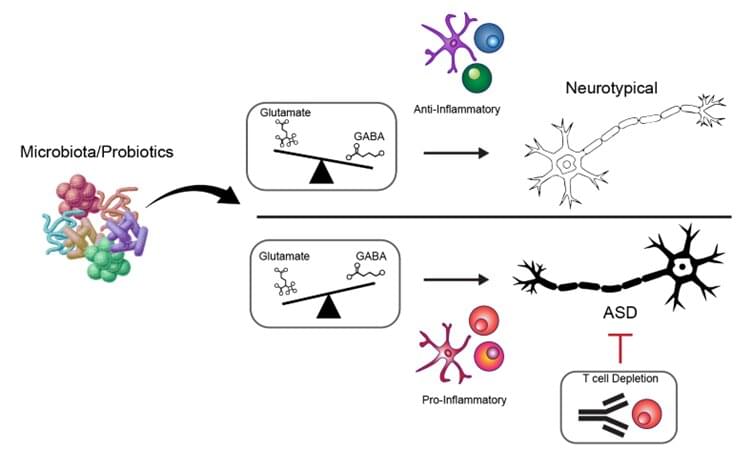
Autism spectrum disorder (ASD) affects an estimated 1 in 31 children in the United States by 2025, and prevalence in East Asian countries, such as South Korea, Singapore, and Japan, may be even higher than those in the United States. Despite its increasing prevalence, the underlying causes of ASD remain poorly understood, and there are currently no curative, preventive, or treatment options available.
A research team from POSTECH and ImmunoBiome in Korea, led by Professor Sin-Hyeog Im, who also serves as the CEO of ImmunoBiome, has made a discovery that reveals a multi-faceted mechanism behind ASD. This study, published in the July issue of Nature Communications, in collaboration with Dr. John C. Park and Prof. Tae-Kyung Kim, demonstrates that the gut microbiota and host immune system together can influence the progression of ASD in a genetic mouse model.
ASD has long been regarded as a genetically driven disorder. However, growing evidence suggests that environmental and microbial factors also play a role. The human gut harbors more than ten times as many microbial cells as human cells, and these microbes play vital roles in metabolism and the development of the immune system.

A new study finds that a high-salt diet triggers brain inflammation that drives up blood pressure. The research, led by McGill University scientist Masha Prager-Khoutorsky in collaboration with an interdisciplinary team at McGill and the Research Institute of the McGill University Health Center, suggests the brain may be a missing link in certain forms of high blood pressure—or hypertension—traditionally attributed to the kidneys.
“This is new evidence that high blood pressure can originate in the brain, opening the door for developing treatments that act on the brain,” said Prager-Khoutorsky, associate professor in McGill’s Department of Physiology.
Hypertension affects two-thirds of people over 60 and contributes to 10 million deaths worldwide each year. Often symptomless, the condition increases the risk of heart disease, stroke and other serious health problems.

3D-Printed Exoskeleton Learns From Your Hand ‘…small electric motors at the principal joints worked the prosthetic framework by means of steel cables…’ — Fritz Leiber, 1968.
Smartwatch Powered By Slime Mold ‘Living protoplasm incorporated into the Ampek F-a2 recording system…’ — Philip K. Dick, 1966.
Carpentopod Walking Table ‘Twoflower’s Luggage, which was currently ambling along on its little legs…’

A software vulnerability checker with the potential to become a repair shop could keep critical computer systems one step ahead.
High-profile cyberattacks, such as the one that compromised British retailer Marks & Spencer’s customer data in April 2025, highlight the need for better ways to detect software vulnerabilities in the computer systems that increasingly control everything, from oil pipelines to hospital records.
To help, an international research team including Khalifa University’s Merouane Debbah, has developed SecureQwen, a smart software checker that automatically detects and flags vulnerabilities for repair. Powered by an AI model trained in the language of computer code, SecureQwen could even identify weaknesses that it had not explicitly been taught or come upon before.
Few people are aware of Marvel Whiteside Parsons (a.k.a Jack Parsons), co-founder of NASA’s Jet Propulsion Laboratories. Parsons made major contributions to rocket development, particularly in the area of solid fuel propellant. The solid motors on the Space Shuttle and the motors in the Minuteman missile were based on the solid propellant technology that he invented. He was a founding member of Aerojet Corporation, and he even has a crater on the dark side of the moon named after him. So why isn’t he as celebrated as the other founding fathers of spaceflight?
Aleister Crowley, born Edward Alexander Crowley, was a British occultist, writer, mountaineer, philosopher, poet, mystic, drug experimenter, and chess player. He was an influential member in several occult organizations, including the Golden Dawn, the A∴A∴, and Ordo Templi Orientis (O.T.O.), and is best known today for his occult writings, especially The Book of the Law, the central sacred text of Thelema. He gained much notoriety during his lifetime, and was infamously dubbed \.