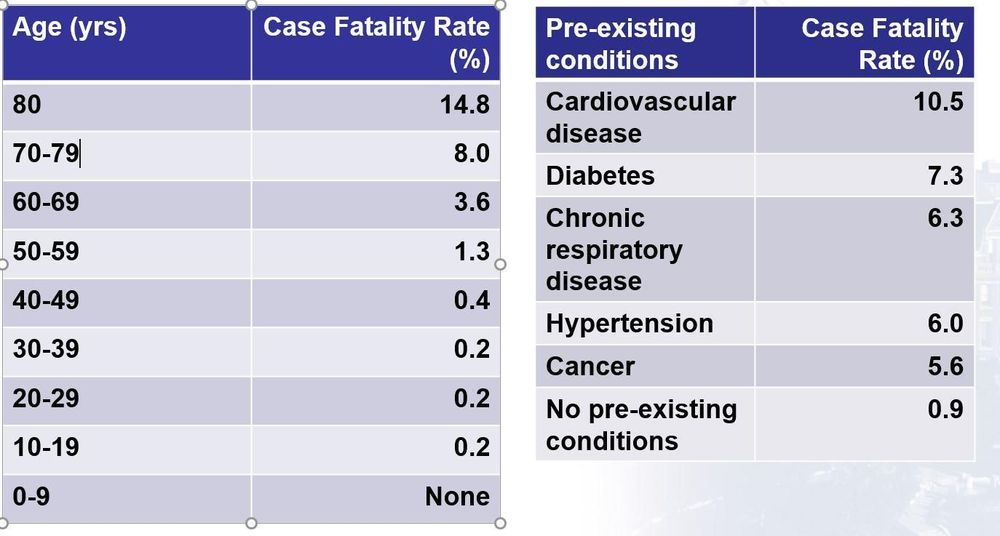
Doctor groups are recommending testing and isolation for people who lose their ability to smell and taste, even if they have no other symptoms.




Covid-19 lasted for 17 days in the cabins of cruise ships:
Cruise ships are often settings for outbreaks of infectious diseases because of their closed environment and contact between travelers from many countries.
What is added by this report?
More than 800 cases of laboratory-confirmed COVID-19 cases occurred during outbreaks on three cruise ship voyages, and cases linked to several additional cruises have been reported across the United States. Transmission occurred across multiple voyages from ship to ship by crew members; both crew members and passengers were affected; 10 deaths associated with cruise ships have been reported to date.
With more than 39,000 Americans infected with coronavirus, the US is now the third hardest-hit country in the world. New York alone has more than 20,000 cases, with 12,305 in the Big Apple.

Last month, even as the coronavirus epidemic was ravaging China and making inroads in other nations, the space industry’s concerns were elsewhere. There were debates about a NASA authorization bill in the House that would reshape NASA’s Artemis program even as the agency sought more money for it, the ongoing review into the flawed test flight of Boeing’s CST-100 Starliner commercial crew vehicle, renewed concerns about orbital debris after a close call between two defunct satellites, and discussions about the viability and sustainability of satellite constellations like OneWeb and SpaceX’s Starlink as both moved into full-scale deployment.
Those were the days. In the last couple of weeks, and especially in the last week, those issues have largely disappeared as what is now a pandemic takes hold in the United States and many other nations. But while many parts of the economy have ground to a halt, like retail and tourism, the effects on the space industry have been uneven. Some parts of it have also effectively halted, yet others continue ahead at essentially full speed—at least for now.
The first clear signs of the effects of the pandemic on the industry was bringing the circuit of conferences and other events to a standstill. On March 9, the Satellite 2020 conference got underway in Washington despite growing concerns about the spread of the coronavirus disease COVID-19, including the first cases diagnosed in the city. Conference organizers plowed ahead even as some major companies, like satellite operator SES, bowed out, saying only about 10 percent of attendees as 12 percent of exhibitors had cancelled their plans.

The emergence of the novel coronavirus and its associated disease, COVID-19, has led to a global pandemic and a call for individuals, in the name of overall public health and an attempt to prevent national medical systems from being overwhelmed with too many patients at once, to self-isolate, self-quarantine, and practice social distancing. Many of us are confronted, for perhaps the only time in our lives, with an uncertain span of time in solitude.
Although this is the first time we’ve seen this particular phenomenon, social distancing isn’t a new invention. Humans have always had good reasons to withdraw from society, often for the greater good. Among the champions of isolation and social distancing are astronauts and cosmonauts—including the late Al Worden—whose time in space has often been spent in extended periods of cramped loneliness, away from family and friends. They can serve as inspiration in these difficult times.
As a cultural anthropologist, my research focuses on human behavior, particularly types of behavior shared by groups, and it is clear that social distancing is unusual. Human beings are gregarious creatures and we do tend to be found in “corporate bodies,” ranging from small bands of 30 to 50 people all the way up to huge cities filled with millions. In band societies, everyone knows everyone else and there’s generally shared work, shared play, and lots of shared gossip. In larger societies, where we may be surrounded by virtual strangers, celebrities seem to fill in as the people everyone knows, giving us membership in a community where celebrities are “shared points of reference” (Hermes and Kooijmann 2016). I may not know you well, but we can probably talk to each other about Sigourney Weaver and Tom Hanks.
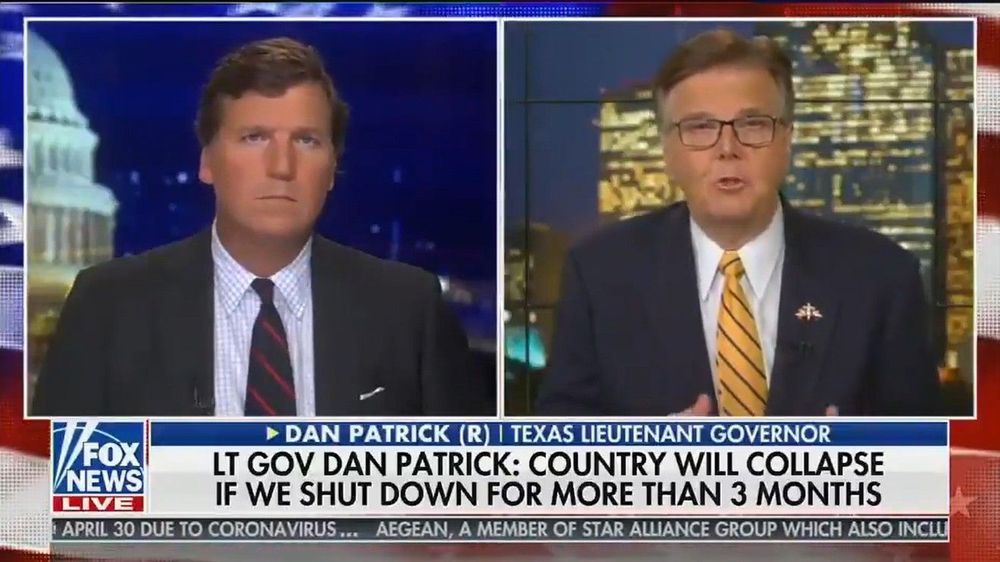
#NotDying4WallStreet
Hard to believe that anyone is this cold-hearted.
As the coronavirus outbreak batters the economy and businesses close, Texas Lt. Gov. Dan Patrick said Monday that plenty of seniors would be willing to sacrifice their lives in order to preserve the economy for their grandchildren.
““No one reached out to me and said, as a senior citizen, are you willing to take a chance on your survival in exchange for keeping the America that all America loves for your children and grandchildren? And if that’s the exchange, I’m all in.””
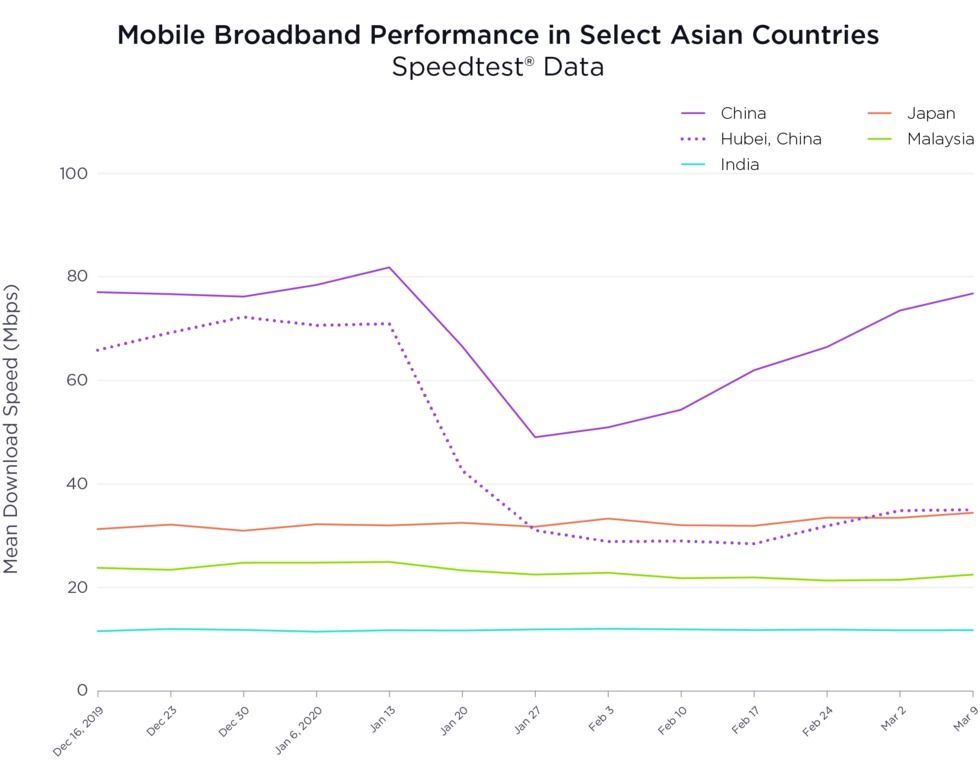
Covid-19 and facemasks.
According to WHO:
If you are healthy, you only need to wear a mask if you are taking care of a person with suspected 2019-nCoV infection.
Wear a mask if you are coughing or sneezing.