If you think tracking apps will keep people safe as economies reopen, look to South Korea, Singapore, and Australia to see why you’re mistaken.




As expected, they discovered large fluctuations in the composition and daily changes of the human and mouse gut microbiomes. But strikingly, these apparently chaotic fluctuations followed several elegant ecological laws.
“Similar to many animal ecologies and complex financial markets, a healthy gut microbiome is never truly at equilibrium,” Vitkup says. “For example, the number of a particular bacterial species on day one is never the same on day two, and so on. It constantly fluctuates, like stocks in a financial market or number of animals in a valley, but these fluctuations are not arbitrary. In fact, they follow predictable patterns described by Taylor’s power law, a well-established principle in animal ecology that describe how fluctuations are related to the relative number of bacteria for different species.”
Other discovered laws of the gut microbiome also followed principles frequently observed in animal ecologies and economic systems, including the tendency of gut bacteria abundances to slowly but predictably drift over time and the tendency of species to appear and disappear from the gut microbiome at predictable times.
“It is amazing that microscopic biological communities—which are about six orders of magnitude smaller than macroscopic ecosystems analyzed previously—appear to be governed by a similar set of mathematical and statistical principles,” says Vitkup.
Laws allow identification of abnormal bacterial behavior.
These universal principles should help researchers to better understand what processes govern the microbial dynamics in the gut. Using the statistical laws, the Columbia researchers were able to identify particular bacterial species with abnormal fluctuations. These wildly fluctuating bacteria were associated with documented periods of gut distress or travel to foreign countries in humans providing data for the study. Thus, this approach may immediately allow researchers to understand and identify which specific bacteria are out of line and behave in a potentially harmful fashion.
Using mice data, the researchers also observed that microbiomes associated with unhealthy high fat diets drift in time significantly faster compared with microbiomes feeding on healthier high fiber diets. This demonstrates that ecological laws can be applied to understand how various dietary changes may affect and perhaps alleviate persistent microbiome instabilities.


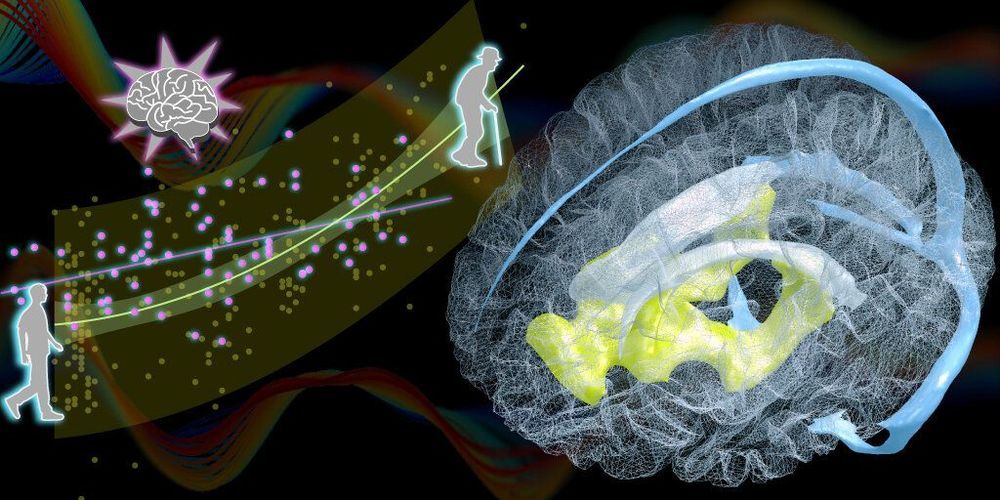
Researchers at the RIKEN Center for Biosystems Dynamics Research (BDR) in Japan have identified changes in the aging brain related to blood circulation. Published in the scientific journal Brain, the study found that natural age-related enlargement of the ventricles—a condition called ventriculomegaly—was associated with a lag in blood drainage from a specific deep region of the brain. The lag can be detected easily with MRI, making it a potential biomarker for predicting ventriculomegaly and the aging brain, which can then be treated quickly.
Ventriculomegaly is an abnormal condition in which fluid accumulates in the ventricles of the brain without properly draining, making them enlarged. Although ventricular enlargement within normal range is not itself considered a disease, when left unchecked it can lead to ventriculomegaly and dementia resulting from normal pressure hydrocephalus. In their study, the team found that ventriculomegaly was associated with changes in blood circulation of the brain. “We found an age-related perfusion timing shift in the brain’s venous systems whose lifespan profile was very similar to, but slightly preceded that of ventricular enlargement,” explains first author Toshihiko Aso.
After blood circulates through the brain providing necessary oxygen, the deoxygenated blood must return to the heart though our veins. This happens through two pathways, one draining blood from regions close to the surface of the brain, and the other from areas deep in the brain. By using MRI to measure changes in blood flow, the team at BDR recently found that as we age, the time it takes for blood to drain through these two pathways becomes out of sync. The result is a time lag between the deep drainage pathway and the surface pathway, which increases with age.

TEL AVIV, Israel, April 22, 2020 /PRNewswire/ — Prof. Dedi (David) Meiri, Chairman and CSO of CannaSoul and Nadav Eyal, Co-founder and CEO of Eybna Technologies Ltd, announced today the companies have jointly engaged in a mutual assays of CannaSoul’s (through its Myplant-Bio subsidiary) Cytokine Storm Assay and Eybna’s Novel NT-VRL™ formulation dedicated for treatment and prevention of viral infections — specifically for high-risk populations and treatment of actively ill patients.

https://sc.mp/subscribe-youtube
While nightclubs have closed in Germany as part of its coronavirus restrictions, a club held the country’s first drive-in social-distancing rave on May 9, 2020.
Follow us on:
Website: https://scmp.com
: https://facebook.com/scmp
: https://twitter.com/scmpnews
Instagram: https://instagram.com/scmpnews
: https://www.linkedin.com/company/south-china-morning-post/