A new artificial intelligence tool will help Stanford Health Care physicians inform patients of their test results, with the goal of reducing administrative tasks.


A new artificial intelligence tool will help Stanford Health Care physicians inform patients of their test results, with the goal of reducing administrative tasks.

Results from a randomized controlled trial reveal that vitamin D supplementation helps maintain telomeres, protective caps at the ends of chromosomes that shorten during aging and are linked to the development of certain diseases.
The new report, which is published in The American Journal of Clinical Nutrition, is based on data from a VITAL (VITamin D and OmegA-3 TriaL) sub-study co-led by researchers at the Harvard-affiliated Mass General Brigham and the Medical College of Georgia, and supports a promising role in slowing a pathway for biological aging.
“VITAL is the first large-scale and long-term randomized trial to show that vitamin D supplements protect telomeres and preserve telomere length,” said co-author JoAnn Manson, the principal investigator of VITAL and chief of the Division of Preventive Medicine at Harvard-affiliated Brigham and Women’s Hospital and the Michael and Lee Bell Professor of Women’s Health at Harvard Medical School.
Trial shows protection against telomere shortening, which heightens disease risk.
Paris, France, Cambridge, USA, June 24, 2025 – TISSIUM, a MedTech company pioneering biomorphic programmable polymers for tissue reconstruction, today announced that the U.S. Food and Drug Administration (FDA) has granted De Novo marketing authorization for COAPTIUM® CONNECT with TISSIUM Light, a first-of-its-kind atraumatic sutureless solution for peripheral nerve repair.
This authorization represents a pivotal regulatory milestone for TISSIUM, further validating its biopolymer platform and enabling U.S. commercialization of its first product. COAPTIUM® CONNECT is now the only FDA-authorized system designed for atraumatic sutureless nerve coaptation. Christophe Bancel, Co-Founder and CEO of TISSIUM said: “This FDA marketing authorization validates over a decade of scientific and clinical commitment to developing next-generation solutions in tissue reconstruction. COAPTIUM® CONNECT is the first demonstration of the transformative potential of our polymer platform and an important step in making atraumatic tissue repair available to patients.”
This regulatory milestone marks TISSIUM’s entry into the U.S. market and establishes the foundation of the TISSIUM polymer platform for atraumatic tissue repair.

In studies with genetically engineered mice, Johns Hopkins Medicine researchers say they have identified a potentially new biological target involving Aplp1, a cell surface protein that drives the spread of Parkinson’s disease-causing alpha-synuclein.
The findings, published May 31 2024 in Nature Communications, reveal how Aplp1 connects with Lag3, another cell surface receptor, in a key part of a process that helps spread harmful alpha-synuclein proteins to brain cells. Those protein buildups are hallmarks of Parkinson’s disease.
Notably, the researchers say, Lag3 is already the target of a combination cancer drug approved by the U.S. Food and Drug Administration (FDA) that uses antibodies to “teach” the human immune system what to seek and destroy.
A novel way to prevent the spread of malaria – a potentially life-threatening disease transmitted through bites from mosquitoes infected by a parasite – could soon be realized, thanks to scientists at The Walter and Eliza Hall Institute of Medical Research (WEHI) in Australia.
While vaccines for malaria exist and more are being formulated for greater efficacy, the WEHI team went right to the source. It first visualized the protein complex that facilitates the reproduction of the Plasmodium falciparum parasite inside mosquitoes. Then, the scientists developed a mRNA vaccine – based on similar technology used for some COVID-19 vaccines – to block the fertilization process. The result: a 99.7% drop in the rate of transmission of the malaria-causing parasite recorded in preclinical studies.
That could be a huge step forward in the fight against this widespread disease, which affects nearly 300 million people around each year, and claims 600,000 lives annually.
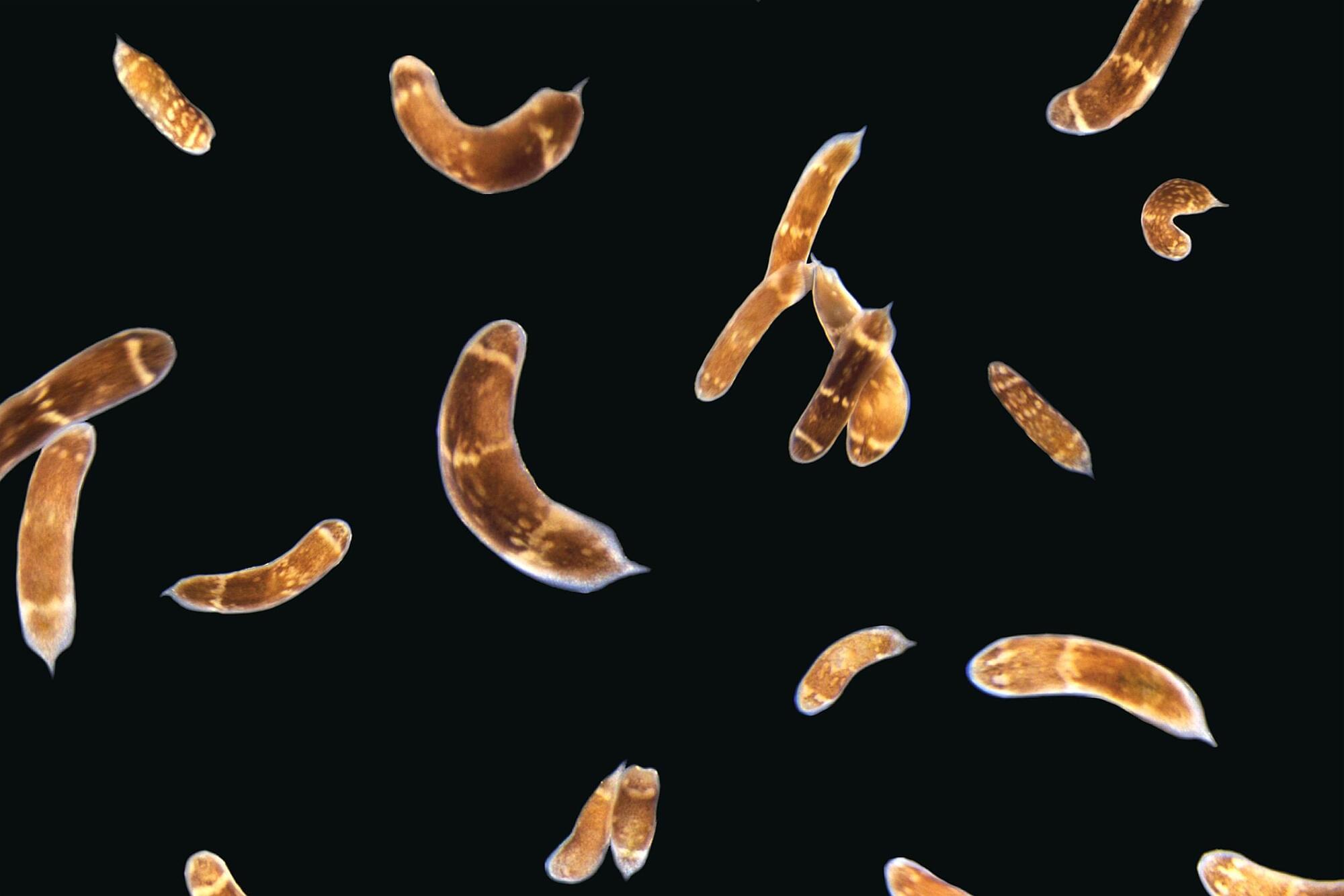
Basically I believe that the flatworm could give the genetic code for essentially brain immortality other just nad plus. But it would have to be made in the brain and controlled possibly with nanotransfection which would scan the body and modify the human brain cells to have its characteristics that may already exist in the human brain also.
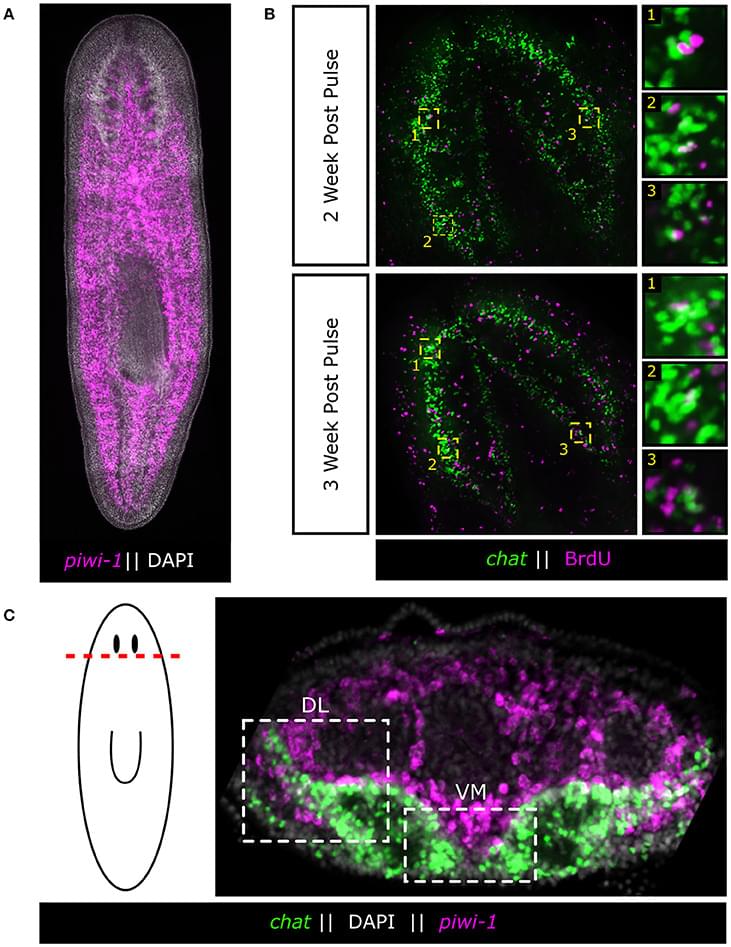
Powerful genetic tools in classical laboratory models have been fundamental to our understanding of how stem cells give rise to complex neural tissues during embryonic development. In contrast, adult neurogenesis in our model systems, if present, is typically constrained to one or a few zones of the adult brain to produce a limited subset of neurons leading to the dogma that the brain is primarily fixed post-development. The freshwater planarian (flatworm) is an invertebrate model system that challenges this dogma. The planarian possesses a brain containing several thousand neurons with very high rates of cell turnover (homeostasis), which can also be fully regenerated de novo from injury in just 7 days. Both homeostasis and regeneration depend on the activity of a large population of adult stem cells, called neoblasts, throughout the planarian body. Thus, much effort has been put forth to understand how the flatworm can continually give rise to the diversity of cell types found in the adult brain. Here we focus on work using single-cell genomics and functional analyses to unravel the cellular hierarchies from stem cell to neuron. In addition, we will review what is known about how planarians utilize developmental signaling to maintain proper tissue patterning, homeostasis, and cell-type diversity in their brains. Together, planarians are a powerful emerging model system to study the dynamics of adult neurogenesis and regeneration.
The adult brain has long been thought to be a fixed structure due to its immense complexity as is illustrated succinctly in the following quote from prominent nineteenth century neuroscientist and Nobel laureate Santiago Ramón y Cajal:
“Once the development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree.”

Scientists have discovered a direct cause-and-effect link between faulty mitochondria and the memory loss seen in neurodegenerative diseases. By creating a novel tool to boost mitochondrial activity in mouse models, researchers restored memory performance, suggesting mitochondria could be a powerful new target for treatments. The findings not only shed light on the early drivers of brain cell degeneration but also open possibilities for slowing or even preventing diseases like Alzheimer’s.