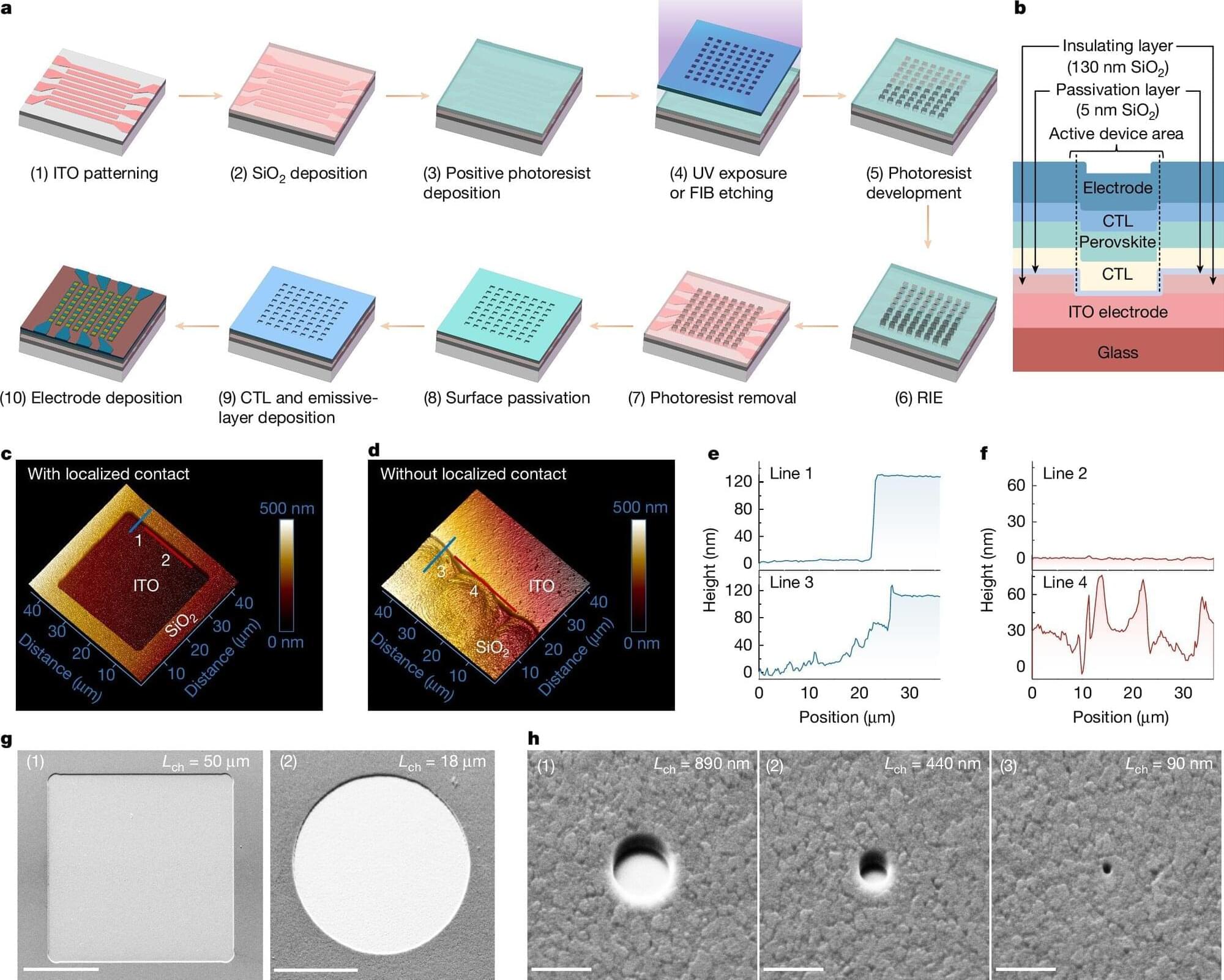
A team of physicists, engineers, opticians and photonics specialists at Zhejiang University, in China, working with a pair of colleagues from the University of Cambridge, in the U.K., has found a way to make pixels smaller by using perovskite. In their paper published in the journal Nature, the group describes how they used the mineral to create pixels as small as a virus.
As the research team notes, the rallying cry for electronics in the modern age is to add more technology to ever smaller base units. For computers, for many years, the goal was to double the number of transistors on a single integrated circuit. Similarly, reducing the size of pixels in video displays has led to sharper and sharper imagery.
The current standard for digital display technology is micro-LED, which is based on II-V semiconductors. Unfortunately, such technology becomes too expensive and inefficient to make pixels any smaller than the size currently in use. This led the team to wonder if a different base material might allow the creation of smaller pixels that would be both cost-effective and efficient. They turned to perovskite, the same mineral that is currently being investigated as a replacement for silicon in solar cells as a way to reduce costs.