Life is almost back to normal in Melbourne, Australia. Here’s how they did it.


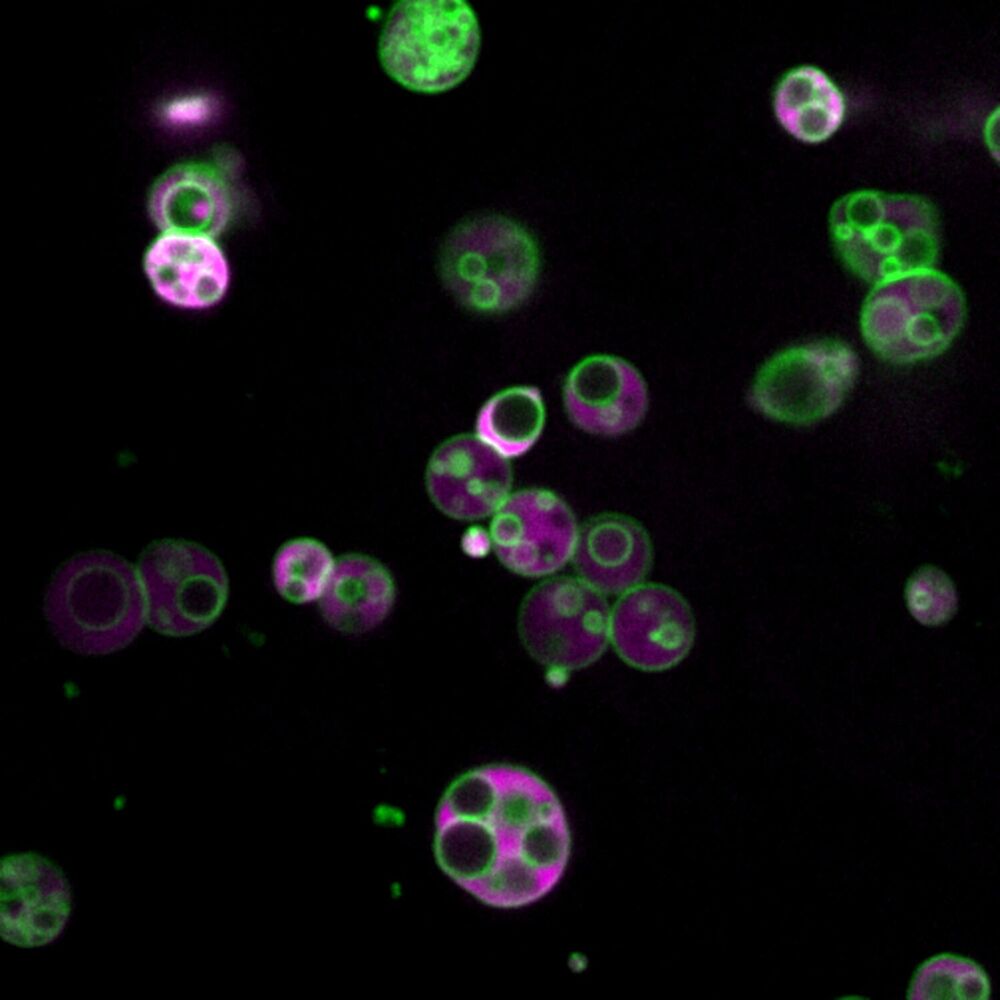
**Peroxisomes are compartments where cells turn fatty molecules into energy and useful materials, like the myelin sheaths that protect nerve cells. In humans, peroxisome dysfunction has been linked to severe metabolic disorders, and peroxisomes may have wider significance for neurodegeneration, obesity, cancer and age-related disorders.**
Peroxisomes are also highly conserved, from plants to yeast to humans, and Bartel said there are hints that these structures may be general features of peroxisomes.
“Peroxisomes are a basic organelle that has been with eukaryotes for a very long time, and there have been observations across eukaryotes, often in particular mutants, where the peroxisomes are either bigger or less packed with proteins, and thus easier to visualize,” she said. But people didn’t necessarily pay attention to those observations because the enlarged peroxisomes resulted from known mutations.
The researchers aren’t sure what purpose is served by the subcompartments, but Wright has a hypothesis.
“When you’re talking about things like beta-oxidation, or metabolism of fats, you get to the point that the molecules don’t want to be in water anymore,” Wright said. “When you think of a traditional kind of biochemical reaction, we just have a substrate floating around in the water environment of a cell—the lumen—and interacting with enzymes; that doesn’t work so well if you’ve got something that doesn’t want to hang around in the water.”
“So, if you’re using these membranes to solubilize the water-insoluble metabolites, and allow better access to lumenal enzymes, it may represent a general strategy to more efficiently deal with that kind of metabolism,” he said.
Bartel said the discovery also provides a new context for understanding peroxisomal disorders.

Exploring the frontiers of neuromodulation, neurostimulation, and neural interfaces.
Neuromodulation is defined as “the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body”. It is carried out to normalize – or modulate – nervous tissue function.
Neuromodulation is an evolving therapy that can involve a range of electromagnetic stimuli such as a magnetic field, an electric current, or a drug instilled directly in the sub-dural space (i.e. intra-thecal drug delivery).
Emerging applications involve targeted introduction of genes or gene regulators and light (optogenetics), but most clinical experience has been with electrical stimulation.
Existing and emerging neuromodulation treatments also include application in medication-resistant epilepsy, chronic head pain conditions, and functional therapy ranging from bladder and bowel or respiratory control, to improvement of sensory deficits, such as hearing and vision.
From ultra high speed levitating trains to lifesaving MRI machines, superconductors are key to some of the world’s most cutting edge technology. But they require extremely low temperatures to work and have remained too expensive for everyday use. Now that could be about to change. With superconductors that work at room temperature, our technological ability is posed to make a giant leap forward.
Check out VICE News for more: http://vicenews.com.
Follow VICE News here:
Facebook: https://www.facebook.com/vicenews.
Twitter: https://twitter.com/vicenews.
Tumblr: http://vicenews.tumblr.com/
Instagram: http://instagram.com/vicenews.
More videos from the VICE network: https://www.fb.com/vicevideo
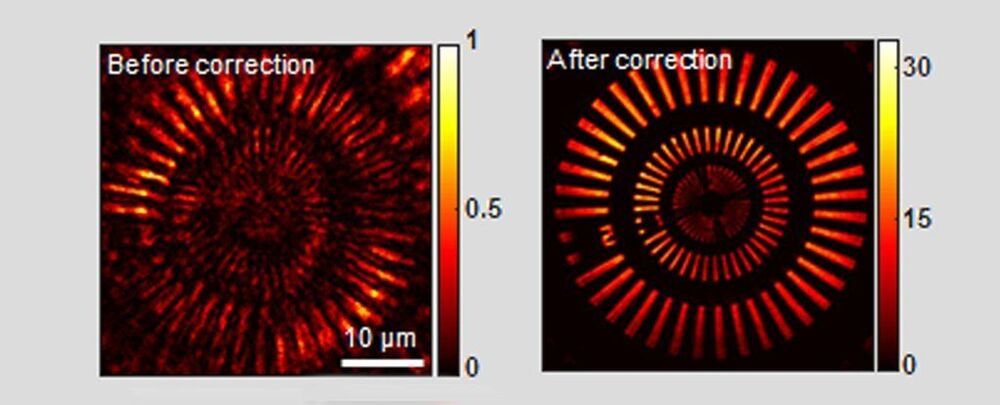
A team of scientists has now found a way to create a clear image from scattered infrared light emitted from a laser, even after it’s passed through a thick layer of bone.
‘Our microscope allows us to investigate fine internal structures deep within living tissues that cannot be resolved by any other means,’ said physicists Seokchan Yoon and Hojun Lee from Korea University.
Seeing what the heck is going on inside of us is useful for many aspects of modern medicine. But how to do this without slicing and dicing through barriers like flesh and bone to observe living intact tissues, like our brains, is a tricky thing to do.
Thick, inconsistent structures like bone will scatter light unpredictably, making it difficult to figure out what’s going on behind them. And the deeper you wish to see, the more scattered light obscures fine and fragile biological structure.
There are plenty of options for researchers who are keen to watch living tissues do their thing, using clever optical tricks to turn scattered photons moving at certain frequencies into an image. But by risking tissue damage or operating only at shallow depths, they all have drawbacks.

Scientists have recovered DNA from a well-preserved horned lark found in Siberian permafrost. The results can contribute to explaining the evolution of sub species, as well as how the mammoth steppe transformed into tundra, forest and steppe biomes at the end of the last Ice Age.
In 2018, a well-preserved frozen bird was found in the ground in the Belaya Gora area of north-eastern Siberia. Researchers at the Centre for Palaeogenetics, a new research center at Stockholm University and the Swedish Museum of Natural History, haves studied the bird and the results are now published in the scientific journal Communications Biology. The analyses reveals that the bird is a 46,000-year-old female horned lark.
“Not only can we identify the bird as a horned lark. The genetic analysis also suggests that the bird belonged to a population that was a joint ancestor of two sub species of horned lark living today, one in Siberia, and one in the steppe in Mongolia. This helps us understand how the diversity of sub species evolves,” says Nicolas Dussex, researcher at the Department of Zoology at Stockholm University.

The proposal would end a federal ban on marijuana and create a pathway to expunge related criminal records.
As the cannabis industry continues to take root state by state, the House of Representatives voted in favor of removing marijuana from the federal Controlled Substances Act.
The House voted Friday on the Marijuana Opportunity Reinvestment and Expungement Act, or MORE Act, which decriminalizes cannabis and clears the way to erase nonviolent federal marijuana convictions. The Senate is unlikely to approve the bill.
The MORE Act also creates pathways for ownership opportunities in the emerging industry, allows veterans to obtain medical cannabis recommendations from Veteran Affairs doctors, and establishes funding sources to reinvest in communities disproportionately affected by the war on drugs.
Dr leonard hayflick — father of cell senescence!
Dr. Leonard Hayflick, is Professor of Anatomy, University of California, San Francisco School of Medicine, where he has been part of the faculty since 1988.
Dr. Hayflick received his Ph.D. at the University of Pennsylvania, did a post-doctoral fellowship at the University of Texas under the tutelage of the renowned cell culturist Prof. Charles Pomerat, and then returned to Philadelphia, where he spent ten years as an Associate Member of the Wistar Institute, and two years as an Assistant Professor of Research Medicine at the University of Pennsylvania.
Dr. Hayflick is extremely well known for his research in a range of domains including cell biology, virus vaccine development, and mycoplasmology.
In 1962 he discovered that, contrary to what was believed since the turn of the century, cultured normal human and animal cells have a limited capacity to replicate. This phenomenon became known as “The Hayflick Limit” which became a discovery that overturned a dogma that existed since early in the twentieth century and focused attention on the cell as the fundamental location of age changes.
