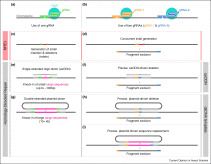
Initially discovered in bacteria, CRISPR-based genome editing endonucleases have proven remarkably amenable for adaptation to insects. To date, these endonucleases have been utilized in a plethora of both model and non-model insects including diverse flies, bees, beetles, butterflies, moths, and grasshoppers, to name a few, thereby revolutionizing functional genomics of insects. In addition to basic genome editing, they have also been invaluable for advanced genome engineering and synthetic biology applications. Here we explore the recent genome editing advancements in insects for generating site-specific genomic mutations, insertions, deletions, as well as more advanced applications such as Homology Assisted Genome Knock-in (HACK), potential to utilize DNA base editing, generating predictable reciprocal chromosomal translocations, and development gene drives to control the fate of wild populations.