Researchers have discovered hepatitis C virus (HCV) in the brain lining of individuals with schizophrenia and bipolar disorder, suggesting a possible link between infection and psychiatric symptoms.

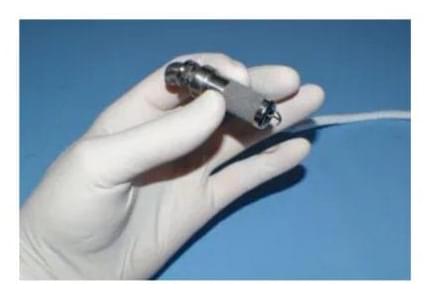
A new type of surgically implanted pump that can support a child’s failing heart has passed the first stage of human testing in a Stanford Medicine-led trial.

Oligonucleotide therapies — engineered strands of DNA or RNA — are transforming modern medicine. These cutting-edge treatments bring a new level of precision in combating disease by targeting specific genes to be silenced, activated or edited. “Nucleotide therapeutics allow us to design predictable outcomes by modifying sequences to address almost any condition,” says Peter Guterstam, product manager at biotechnology company Cytiva.
Due to an influx of research in recent years, many nucleotide-based drug candidates, including genetic therapies and vaccines for cancer and viral infections, are now in advanced clinical trial stages. “The development timeline is much quicker than we are used to,” notes Guterstam.
Significant challenges arise during development of RNA and DNA based therapies. From mRNA vaccines to gene editing, scientists are refining delivery methods, optimizing synthesis, and tackling scaling hurdles.


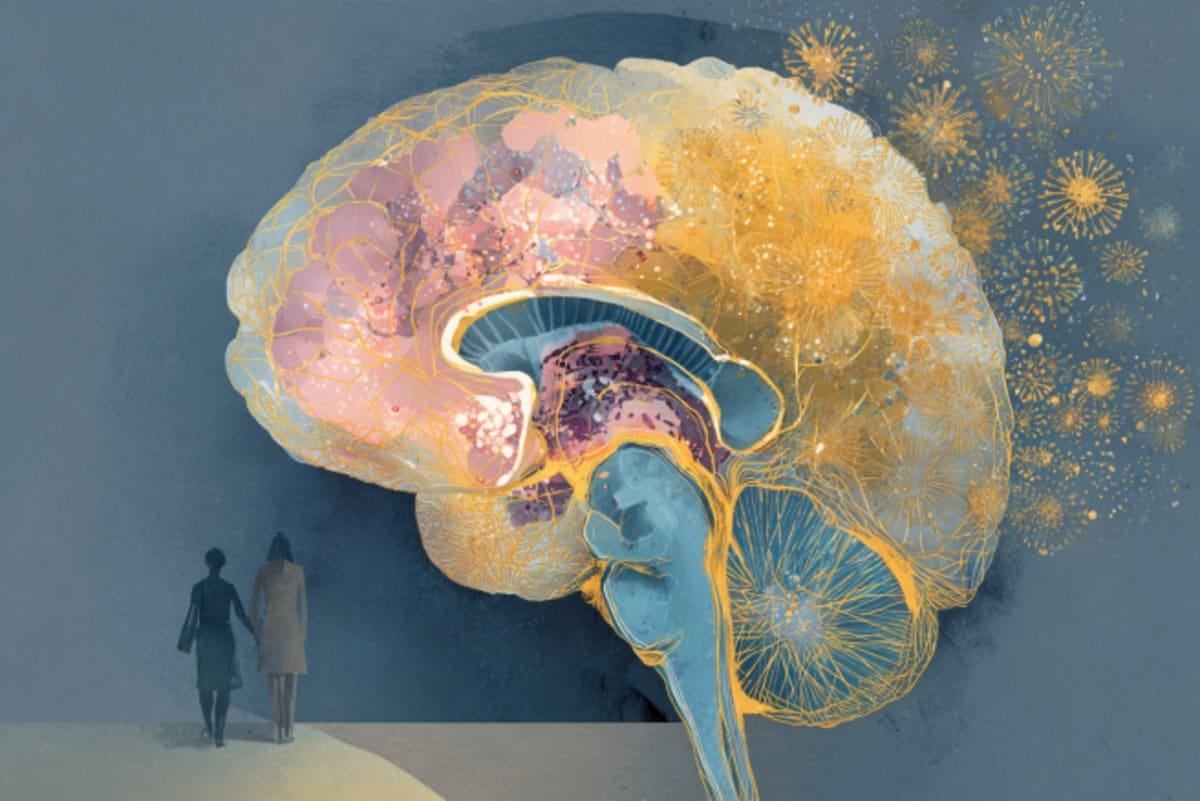
A person will have Alzheimer’s years before ever knowing it. The disorienting erasure of memories, language, thoughts—in essence, all that makes up one’s unique sense of self—is the final act of this enigmatic disease that spends decades disrupting vital processes and dismantling the brain’s delicate structure.
Once symptoms surface and doctors make a diagnosis, though, it can often be too late. Damage is widespread, impossible to reverse. No cure exists.
Attempts to develop drugs that clear away toxic accumulations of amyloid-beta and tau proteins—hallmarks of the disease that cause neurons to die—have ended in hundreds of failed clinical trials. Today, some scientists are skeptical over whether removing amyloid plaques is even enough. Others have a hunch that the best line of attack won’t target just one aspect of the disease, but many of them, all at once.
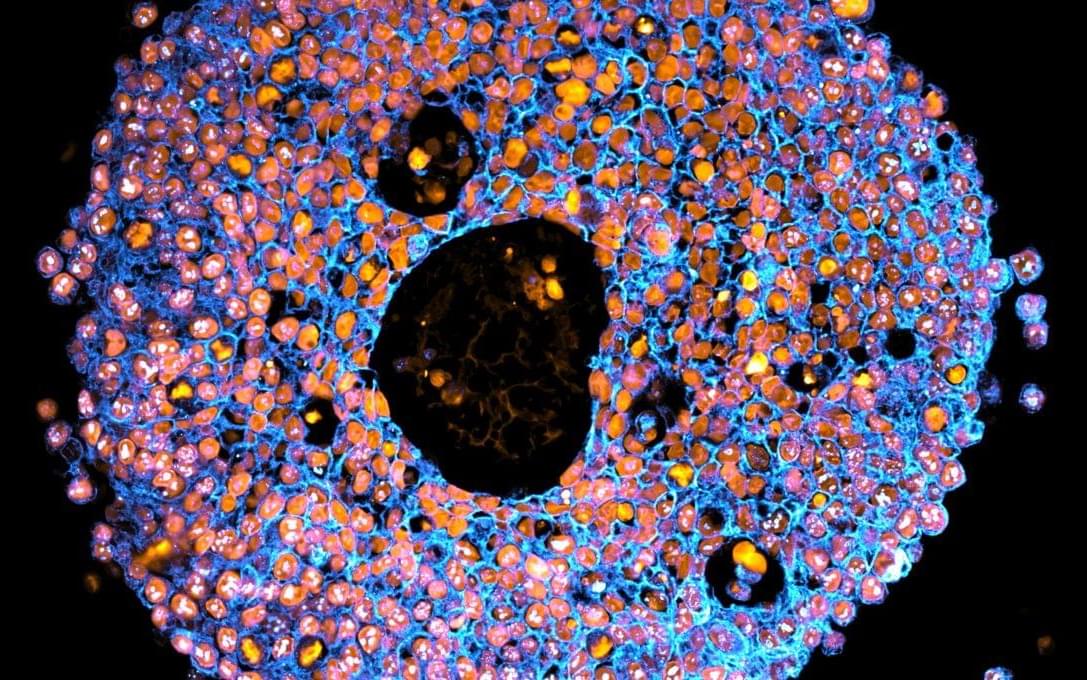
To address these shortcomings, the team behind the latest study turned to bioprinting – a type of 3D printing that uses living cells and cell-friendly materials to create 3D structures. They took trophoblast cells and mixed them with a synthetic gel before 3D-printing them in precise droplets.
The printed cells then grew into miniature placentas, and the researchers compared them to organoids made via traditional manual methods.
“The organoids we grew in the bioprinted gel developed differently to those grown in an animal-derived gel, and formed different numbers of trophoblast sub-types. This highlighted that the environment organoids are grown in can control how they mature,” first author Dr Claire Richards said.

The dietary supplement nicotinamide has been recommended by dermatologists for people with a history of skin cancer since 2015, when a clinical study with 386 participants showed that those who took the vitamin B3 derivative developed fewer new occurrences.
However, data to validate those findings in a larger study group has been lacking because nicotinamide can be purchased over the counter without being entered into patients’ medical records. In a new study published Sept. 17 in JAMA Dermatology, researchers found a way to get that data by analyzing records from the Veterans Affairs Corporate Data Warehouse.
Nicotinamide is on the VA’s official formulary, so the researchers checked the outcomes of 33,833 patients for their next skin cancer diagnosis following baseline treatment with 500 milligrams of nicotinamide twice daily for longer than 30 days. They looked for occurrences of basal cell carcinoma and cutaneous squamous cell carcinoma.

Stanford Medicine researchers trained a large language model to read medical charts, looking for signs that kids with ADHD received the right follow-up care when using new medications.


Targeting therapeutic nanoparticles to the brain poses a challenge due to the restrictive nature of the blood–brain barrier (BBB). Here we report the development of mRNA-loaded lipid nanoparticles (LNPs) functionalized with BBB-interacting small molecules, thereby enhancing brain delivery and gene expression. Screening brain-targeted mRNA-LNPs in central nervous system (CNS) in vitro models and through intravenous administration in mice demonstrated that acetylcholine-conjugated LNPs achieved superior brain tropism and gene expression, outperforming LNP modifications with nicotine, glucose, memantine, cocaine, tryptophan, and other small molecules. An artificial intelligence (AI)-based model designed to predict the BBB permeability of small-molecule ligands showed strong alignment with our experimental results, providing in vivo validation of its predictive capacity. Cell-specific biodistribution analysis in Cre-reporter Ai9 mice showed that acetylcholine-functionalized LNPs preferentially transfected neurons and astrocytes following either intravenous or intracerebral administration. Mechanistic studies suggest that acetylcholine-LNP uptake is mediated by the functional engagement of acetylcholine receptors (AchRs) followed by endocytosis, which synergistically enhances intracellular mRNA delivery. Moreover, acetylcholine-LNPs successfully crossed a human BBB-on-a-chip model, enabling transgene expression in human iPSC-derived neurons. Their effective penetration and transfection in human brain organoids further support their potential activity in human-based systems. These findings establish a predictive and modular framework for engineering CNS-targeted LNPs, advancing precision gene delivery for brain disorders.