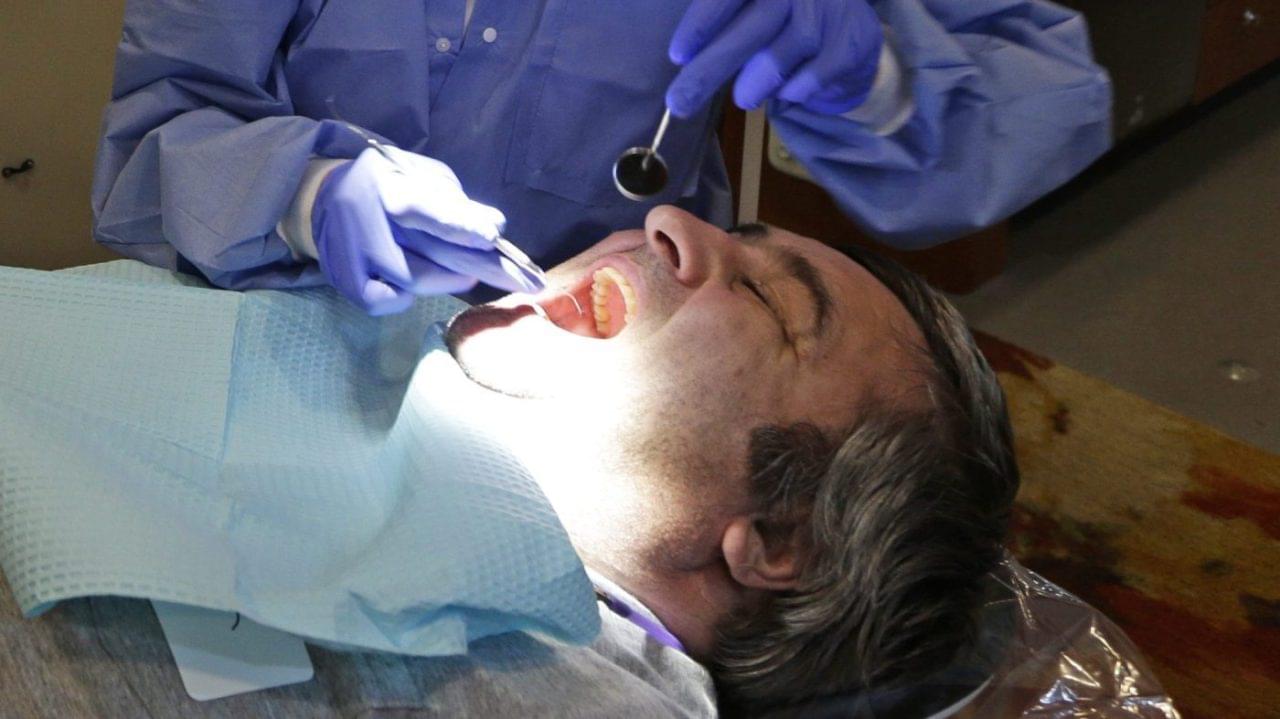
Dentists warn they’re seeing cases of something nicknamed “Ozempic teeth.” Medications like Ozempic and Wegovy can lead to dry mouth because the active ingredient, semaglutide, affects the salivary glands, explains Adam Taylor, an anatomy professor at Lancaster University, in an article for The Conversation. The medications can also cause people to drink less water because they feel less thirsty.
Those factors combined increase the risk of cavities and gum disease, explained Dr. Rajpal Anjali, a cosmetic dentist at Beverly Hills Dental Arts.
To make things worse, some people also experience side effects like acid reflux and vomiting, further harming their tooth enamel.