Acute Type A Aortic Dissection (aTAAD) is a severe and life-threatening condition. While animal studies have suggested that ketorolac could slow the progression of aortic aneurysms and dissections, clinical data on its efficacy in aTAAD patients remain limited. This study seeks to evaluate the safety and effectiveness of ketorolac in this patient group.
Patients were randomly assigned to receive either ketorolac or a placebo (0.9% saline). Treatment began at least 2 h prior to surgery (60 mg ketorolac or 2 ml saline administered once intramuscularly) and continued for 48 h post-surgery (30 mg ketorolac or 1 ml saline administered intramuscularly twice daily). The primary endpoints included assessing the safety and efficacy of ketorolac in improving the prognosis of aTAAD, focusing on mortality and organ malperfusion syndrome. Secondary endpoints included drug-related adverse events, blood test results, and other postoperative outcomes.
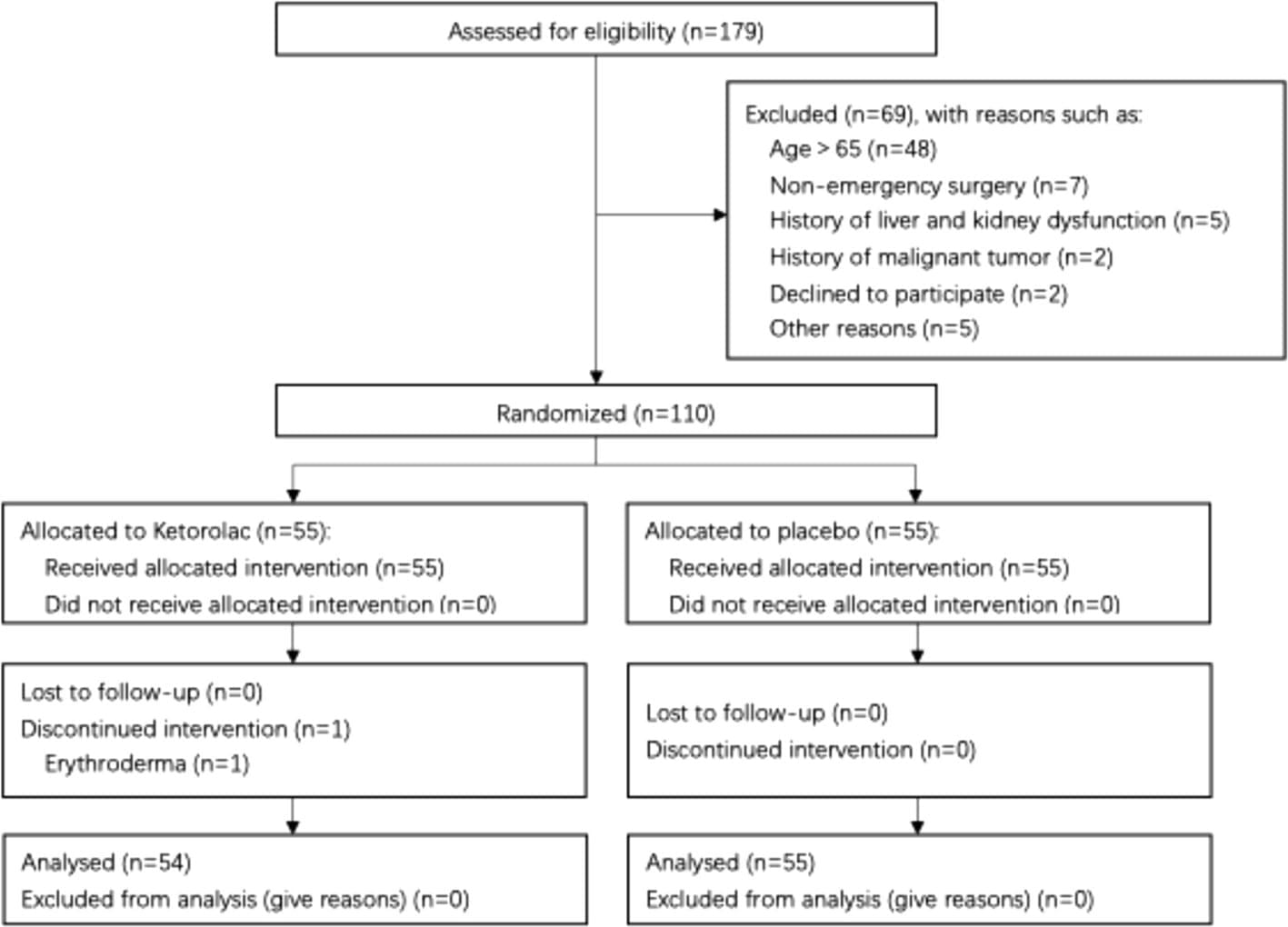
Of 179 patients who underwent aTAAD repair, 110 met the inclusion criteria and were randomized into two groups of 55. One patient discontinued the intervention due to erythroderma on the first postoperative day, leaving 54 patients in the ketorolac group and 55 in the placebo group for analysis. No significant differences were found in the primary endpoints. However, the ketorolac group showed lower intraoperative bleeding (median: 1.8 L vs. 2.0 L, P = 0.03), shorter intensive care unit (ICU) stays (median: 6.5 days vs. 8 days, P = 0.04), and lower total hospital costs (median: ¥170,430 vs. ¥187,730, P = 0.03).